Suzuki–Miyaura Coupling
Synthesis of Biaryls through Nickel-Catalyzed Suzuki–Miyaura Coupling of Amides by Carbon–Nitrogen Bond Cleavage (pages 6959–6963)Shicheng Shi, Guangrong Meng and Prof. Dr. Michal Szostak
Version of Record online: 21 APR 2016 | DOI: 10.1002/anie.201601914
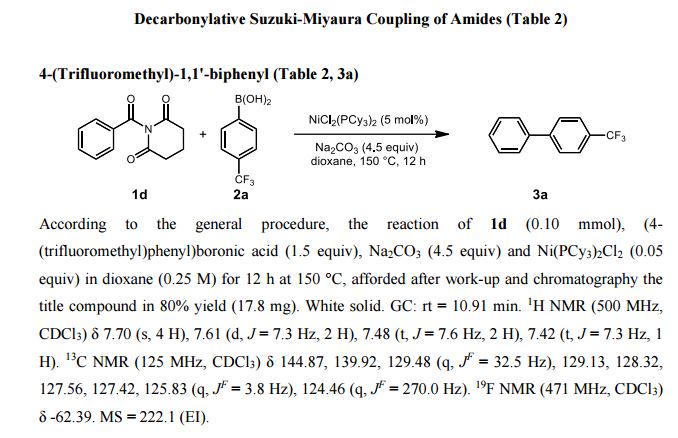
Breaking and making: The first nickel-catalyzed Suzuki–Miyaura coupling of amides for the synthesis of biaryl compounds through N−C amide bond cleavage is reported. The reaction tolerates a wide range of sensitive and electronically diverse substituents on both coupling partners.
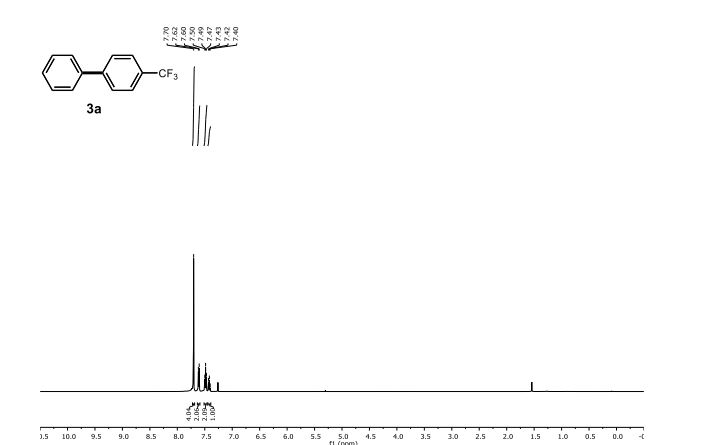
1H NMR (500 MHz, CDCl3) δ 7.70 (s, 4 H), 7.61 (d, J = 7.3 Hz, 2 H), 7.48 (t, J = 7.6 Hz, 2 H), 7.42 (t, J = 7.3 Hz, 1 H).
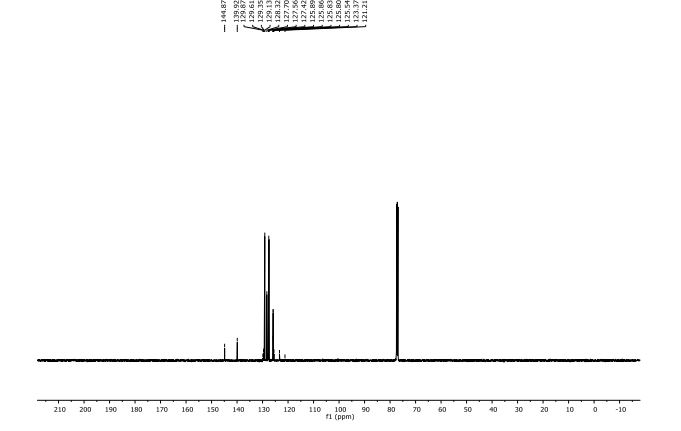
13C NMR (125 MHz, CDCl3) δ 144.87, 139.92, 129.48 (q, J F = 32.5 Hz), 129.13, 128.32, 127.56, 127.42, 125.83 (q, J F = 3.8 Hz), 124.46 (q, J F = 270.0 Hz).
//////Nickel-Catalyzed, Decarbonylative Suzuki–Miyaura Coupling, Amides, Biaryls