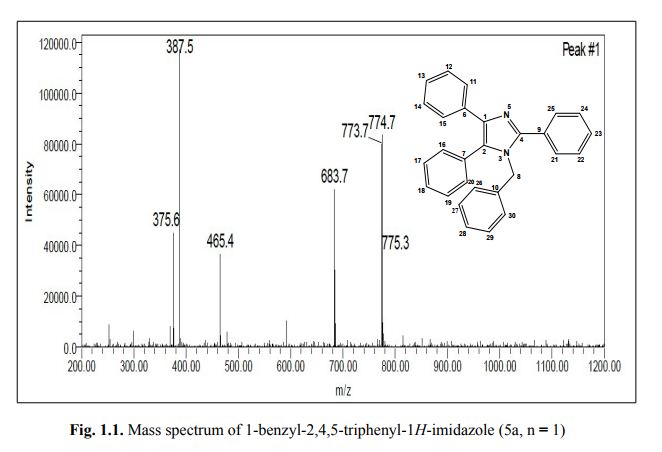
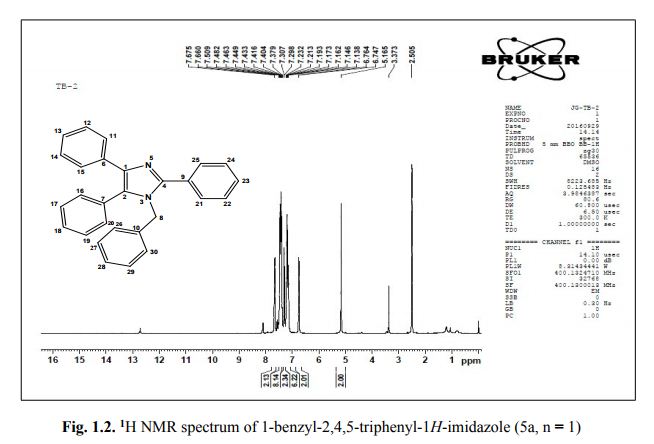
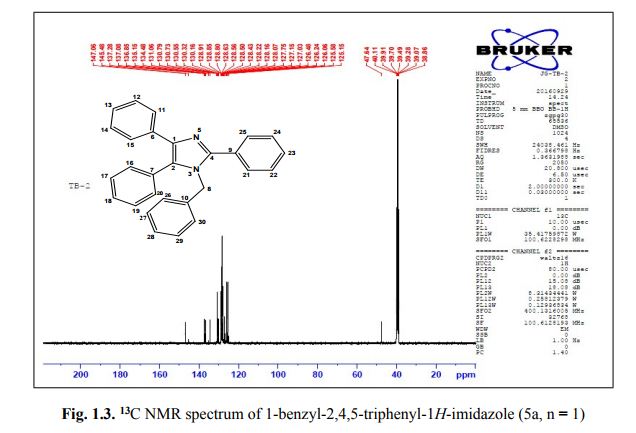
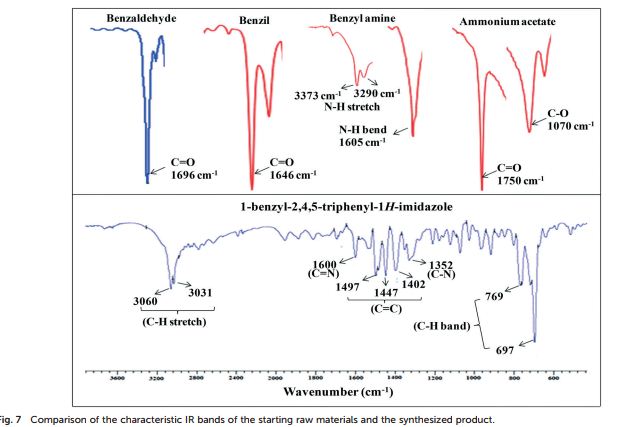
1-benzyl-2, 4, 5-triphenyl-1H-imidazole
. 1-Benzyl-2,4,5-triphenyl-1H-imidazole (5a, n = 1).
Off-white solid; m.p.: 160–162 °C;
anal. calcd. for C28H22N2: C, 87.01, H, 5.74, N, 7.25%. Found: C, 87.13, H, 5.70, N, 7.19%;
UV (λmax, ethanol) = 280 nm;
FT-IR (KBr, cm−1 ): 3060 (C–H stretch), 3031, 1600 (CN), 1497, 1483, 1447 (CC), 1352 (C–N stretch), 769, 697 (C–H band);
1 H NMR (400 MHz, DMSO): 5.16 (s, 2H, CH2), 6.74–7.67 (m, 20H, Ar–H) ppm;
13C NMR (100 MHz, DMSO): 47.6 (CH2, C8), 125.1 (CHarom, C28), 126.0 (CHarom, C26), 126.2 (CHarom, C30), 126.4 (CHarom, C11), 127.0 (CHarom, C15), 127.1 (CHarom, C16), 127.7 (CHarom, C20), 128.0 (CHarom, C21), 128.1 (CHarom, C25), 128.4 (CHarom, C13), 128.5 (CHarom, C18), 128.6 (CHarom, C27), 128.8 (C1), 128.8 (CHarom, C12), 128.9 (CHarom, C14), 130.1 (CHarom, C17), 130.3 (CHarom, C19), 130.5 (CHarom, C22), 130.7 (CHarom, C24), 131.0 (CHarom, C29), 134.4 (CHarom, C9), 135.1 (CHarom, C23), 136.8 (CHarom, C7), 137.0 (CHarom, C10), 137.2 (CHarom, C6), 145.4 (C2), 147.0 (C4) ppm;
MS: m/z = 387.5 (M + H)+
An efficient green protocol for the synthesis of tetra-substituted imidazoles catalyzed by zeolite BEA: effect of surface acidity and polarity of zeolite
*Corresponding authors
Abstract
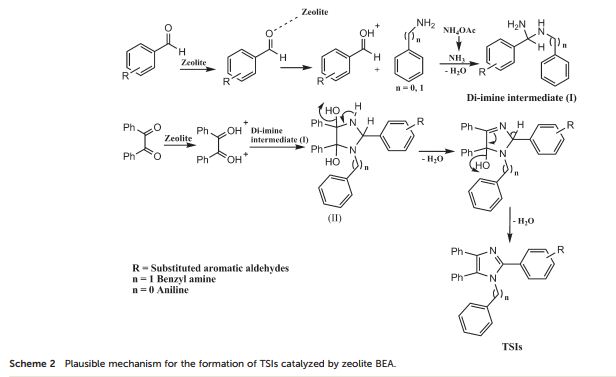
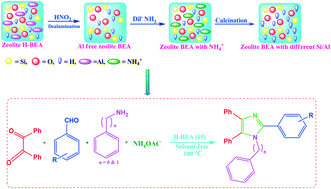
In the present study, the catalytic activity of various medium (H-ZSM-5) and large pore (H-BEA, H-Y, H-MOR) zeolites were studied as solid acid catalysts. The zeolite H-BEA is found to be an efficient catalyst for the synthesis of 1-benzyl-2,4,5-triphenyl-1H-imidazoles through one-pot, 4-component reaction (4-CR) between benzil, NH4OAc, substituted aromatic aldehydes and benzyl amine. The hydrophobicity, Si/Al ratio and acidic properties of zeolite BEA were well improved by controlled dealumination. The synthesized materials were characterized by various characterization techniques such as XRD, ICP-OES, BET, NH3-TPD, FT-IR, pyridine FT-IR, 27Al and 1H MAS NMR. It has been observed that the dealumination of the parent zeolite H-BEA (12) results in the enhanced strength of Brønsted acidity up to a certain Si/Al ratio which is attributed to the inductive effect of Lewis acidic EFAl species, leading to the higher activity of the zeolite BEA (15) catalyst towards the synthesis of 1-benzyl-2,4,5-triphenyl-1H-imidazoles under thermal solvent-free conditions with good to excellent yields. Using the present catalytic synthetic protocol, diverse tetra-substituted imidazoles, which are among the significant biologically active scaffolds, were synthesized in high yield within a shorter reaction time. The effect of polarity, surface acidity and extra framework Al species of the catalysts has been well demonstrated by means of pyridine FT-IR, and 27Al and 1H MAS NMR. The solvent-free synthetic protocol makes the process environmentally benign and economically viable.





////////