
The plant hormone methyl jasmonate can be used to increase broccoli’s antitumoral properties
http://www.chemistryviews.org/details/news/5428251/Boosting_Broccoli_Power.html

back to home for more updates
![]()

DR ANTHONY MELVIN CRASTO Ph.D

The plant hormone methyl jasmonate can be used to increase broccoli’s antitumoral properties
http://www.chemistryviews.org/details/news/5428251/Boosting_Broccoli_Power.html

back to home for more updates
![]()

DR ANTHONY MELVIN CRASTO Ph.D
(NaturalNews) Various cultures around the world already understand that herbs such as ginger, cinnamon, garlic and turmeric can effectively treat conditions like diabetes. According to research from the biomedical science department at the King Faisal University in Saudi Arabia, garlic may be the most powerful of them all for treating diabetes.
Learn more: http://www.naturalnews.com/042941_garlic_diabetes_treatment_oxidative_stress.html##ixzz2l5NBhJ00
Vitamin D is an essential vitamin required by the body for the proper absorption of calcium, bone development, control of cell growth, neuromuscular functioning, proper immune functioning, and alleviation of inflammation. A deficiency in vitamin D can lead to rickets, a disease in which bones fail to properly develop. Further, inadequate levels of vitamin D can lead to a weakened immune system, increased cancer risk, poor hair growth, and osteomalacia, a condition of weakened muscles and bones. Conversely, excess vitamin D can cause the body to absorb too much calcium, leading to increased risk of heart attack and kidney stones. The current U.S. DV for vitamin D is 600 IU (international units) and the toxicity threshold for vitamin D is thought to be 10,000 to 40,000 IU/day.2 Vitamin D is oil soluble, which means you need to eat fat to absorb it. It is naturally found mainly in fish oils, fatty fish, and to a lesser extent in beef liver, cheese, egg yolks, and certain mushrooms. Vitamin D is also naturally made by your body when you expose your skin to the sun, and thus, is called the sun-shine vitamin. In addition, vitamin D is widely added to many foods such as milk and orange juice, and can also simply be consumed as a supplement. Below is a list of high vitamin D foods.
1: Cod Liver Oil
 Cod liver oil has been a popular supplement for many years and naturally contains very high levels of vitamin A and vitamin D. Cod liver oil provides 10001IU (1667% DV) per 100 gram serving, or 1360IU (340% DV) in a single tablespoon.
Cod liver oil has been a popular supplement for many years and naturally contains very high levels of vitamin A and vitamin D. Cod liver oil provides 10001IU (1667% DV) per 100 gram serving, or 1360IU (340% DV) in a single tablespoon.
2: Fish
 Various types of fish are high in vitamin D. Typically raw fish contains more vitamin D than cooked, and fatty cuts will contain more than lean cuts. Further, fish canned in oil will have more vitamin D than those canned in water. Raw fish is typically eaten in the form of sushi. Raw Atlantic Herring provides the most vitamin D with 1628IU (271% DV) per 100 gram serving, 2996IU (499% DV) per fillet, and 456IU (76% DV) per ounce. It is followed by Pickled Herring with 680IU (113% DV) per 100g serving, Canned Salmon (127% DV), Raw Mackerel (60% DV), Oil Packed Sardines (45% DV), Canned Mackerel (42% DV), and oil packed Tuna (39% DV).
Various types of fish are high in vitamin D. Typically raw fish contains more vitamin D than cooked, and fatty cuts will contain more than lean cuts. Further, fish canned in oil will have more vitamin D than those canned in water. Raw fish is typically eaten in the form of sushi. Raw Atlantic Herring provides the most vitamin D with 1628IU (271% DV) per 100 gram serving, 2996IU (499% DV) per fillet, and 456IU (76% DV) per ounce. It is followed by Pickled Herring with 680IU (113% DV) per 100g serving, Canned Salmon (127% DV), Raw Mackerel (60% DV), Oil Packed Sardines (45% DV), Canned Mackerel (42% DV), and oil packed Tuna (39% DV).
3: Fortified Cereals

4: Oysters
 In addition to vitamin D, Oysters are a great source of vitamin b12, zinc, iron, manganese, selenium, and copper. Oysters are also high in cholesterol and should be eaten in moderation by people at risk of heart disease or stroke. Raw wild caught Eastern Oysters provide 320IU (80% DV) per 100 gram serving, 269IU (67% DV) in six medium oysters.
In addition to vitamin D, Oysters are a great source of vitamin b12, zinc, iron, manganese, selenium, and copper. Oysters are also high in cholesterol and should be eaten in moderation by people at risk of heart disease or stroke. Raw wild caught Eastern Oysters provide 320IU (80% DV) per 100 gram serving, 269IU (67% DV) in six medium oysters.
5: Caviar (Black and Red)
 Caviar is a common ingredient in sushi and more affordable than people think. Caviar provides 232IU (58% DV) of vitamin D per 100 gram serving, or 37.1IU (9% DV) per teaspoon.
Caviar is a common ingredient in sushi and more affordable than people think. Caviar provides 232IU (58% DV) of vitamin D per 100 gram serving, or 37.1IU (9% DV) per teaspoon.
6: Fortified Soy Products (Tofu and Soy Milk)
 Fortified soy products are often fortified with both vitamin D and calcium. Fortified Tofu can provide up to 157IU (39% DV) of vitamin D per 100 gram serving, or 44IU (11% DV) per ounce. Fortified Soy Milk can provide up to 49IU (12% DV) of vitamin D per 100 gram serving, 119IU (30% DV) per cup. Amounts of vitamin D vary widely between products, so be sure to check nutrition facts for vitamin D content.
Fortified soy products are often fortified with both vitamin D and calcium. Fortified Tofu can provide up to 157IU (39% DV) of vitamin D per 100 gram serving, or 44IU (11% DV) per ounce. Fortified Soy Milk can provide up to 49IU (12% DV) of vitamin D per 100 gram serving, 119IU (30% DV) per cup. Amounts of vitamin D vary widely between products, so be sure to check nutrition facts for vitamin D content.
7: Salami, Ham, and Sausages
 Salami, Ham, and Sausages are a good source of vitamin b12, and copper. Unfortunately, they are also high in cholesterol and sodium, and so should be limited by people at risk of hypertension, heart attack, and stroke. Salami provides 62.0IU (16% DV) of vitamin D per 100 gram serving, or 16.7IU (4% DV) per ounce (3 slices). It is followed by Bologna Pork 56IU (9% DV) per 100 grams, and Bratwurst 44IU (7% DV) per 100 gram serving.
Salami, Ham, and Sausages are a good source of vitamin b12, and copper. Unfortunately, they are also high in cholesterol and sodium, and so should be limited by people at risk of hypertension, heart attack, and stroke. Salami provides 62.0IU (16% DV) of vitamin D per 100 gram serving, or 16.7IU (4% DV) per ounce (3 slices). It is followed by Bologna Pork 56IU (9% DV) per 100 grams, and Bratwurst 44IU (7% DV) per 100 gram serving.
8: Fortified Dairy Products
 Dairy products are already high in calcium, so it makes sense to fortify them with vitamin D. Milk can provide up to 52.0IU (13% DV) of vitamin D per 100 gram serving, 127IU (32% DV) per cup. Cheese can provide up to 6.6IU (2% DV) in a cubic inch, and butter provides 7.8IU (2% DV) in a single tablespoon. Check nutrition labels for exact amounts.
Dairy products are already high in calcium, so it makes sense to fortify them with vitamin D. Milk can provide up to 52.0IU (13% DV) of vitamin D per 100 gram serving, 127IU (32% DV) per cup. Cheese can provide up to 6.6IU (2% DV) in a cubic inch, and butter provides 7.8IU (2% DV) in a single tablespoon. Check nutrition labels for exact amounts.
9: Eggs
 In addition to vitamin D, eggs are a good source of vitamin B12, and protein. Eggs provide 37.0IU (9% DV) of vitamin D per 100 gram serving, or 17.0IU (4% DV) in a large fried egg.
In addition to vitamin D, eggs are a good source of vitamin B12, and protein. Eggs provide 37.0IU (9% DV) of vitamin D per 100 gram serving, or 17.0IU (4% DV) in a large fried egg.
10: Mushrooms
 More than just a high vitamin D food, mushrooms also provide Vitamin B5 (Pantothenic Acid) and copper. Lightly cooked white button mushrooms provide the most vitamin D with 27.0IU (7% DV) per 100 gram serving, or 7.6IU (2% DV) per ounce.
More than just a high vitamin D food, mushrooms also provide Vitamin B5 (Pantothenic Acid) and copper. Lightly cooked white button mushrooms provide the most vitamin D with 27.0IU (7% DV) per 100 gram serving, or 7.6IU (2% DV) per ounce.
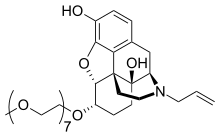
naloxegol
Morphinan-3,14-diol, 4,5-epoxy-6-(3,6,9,12,15,18,21-heptaoxadocos-1-yloxy)-17-(2-
propen-1-yl)-, (5α,6α)-
2. 4,5α-epoxy-6α-[(3,6,9,12,15,18,21-heptaoxadocosan-1-yl)oxy]-17-(prop-2-en-1-
yl)morphinan-3,14-diol
http://www.ama-assn.org/resources/doc/usan/naloxegol.pdf
MOLECULAR FORMULA C34H53NO11
MOLECULAR WEIGHT 651.8
SPONSOR AstraZeneca
CODE DESIGNATION NKTR-118
CAS REGISTRY NUMBER 854601-70-0
The US Food and Drug Administration (FDA) has accepted AstraZeneca’s new drug application (NDA) for naloxegol, an investigational peripherally acting mu-opioid receptor antagonist (PAMORA) for the treatment of opioid-induced constipation (OIC).
read at
FDA accepts AstraZeneca’s new drug application for constipation drug
Naloxegol (INN; NKTR-118), or PEGylated naloxol,[1] is a peripherally-selective opioid antagonist under development by AstraZeneca, licensed from Nektar, for the treatment of opioid-induced constipation.[2]
NALOXEGOL OXALATE

credit kegg
NALOXEGOL OXALATE
http://www.ama-assn.org/resources/doc/usan/naloxegol-oxalate.pdf
Morphinan-3,14-diol, 4,5-epoxy-6-(3,6,9,12,15,18,21-heptaoxadocos-1-yloxy)-
17-(2-propen-1-yl)-, (5α,6α)-, ethanedioate (1:1)
2. 4,5α-epoxy-6α-[(3,6,7,12,15,18,21-heptaoxadocosyl)oxy]-17-(prop-2-
enyl)morphinan-3,14-diol hydrogen ethanedioate
MOLECULAR FORMULA C34H53NO11 . C2H2O4
MOLECULAR WEIGHT 741.8
SPONSOR AstraZeneca
CODE DESIGNATIONS NKTR-118 oxalate, AZ13337019 oxalate
CAS REGISTRY NUMBER 1354744-91-4
About Opioid-Induced Constipation
Opioids are commonly prescribed to patients experiencing chronic pain, which can provide relief from serious medical conditions including osteoarthritis, cancer, and chronic back pain.1 There are about 250 million opioid prescriptions written annually in the US alone to treat these conditions.2 Patients taking opioids to treat chronic pain commonly experience a side effect known as opioid-induced constipation, which may include infrequent bowel movements and difficulty passing stools or emptying bowels.1,3 Clinically, OIC is the most prevalent side effect of opioid therapy.4 For those patients who take opiates for long term pain management, approximately 40-50 percent commonly experience OIC.5 Only about 40-50 percent of those patients experience effective relief from current treatment options.6,7
About Naloxegol (NKTR-118)
Naloxegol (NKTR-118) is an investigational drug candidate in Phase 3 studies being developed as a once-daily oral tablet for the treatment of opioid-induced constipation. Naloxegol (NKTR-118) was designed using Nektar’s proprietary small molecule polymer conjugate technology. Results of the Phase 2 study of naloxegol (NKTR-118) were presented in October 2009 at the American College of Gastroenterology Annual Clinical Meeting and the American Academy of Pain Management. NKTR-119 is an early stage drug development program that is intended to combine oral naloxegol (NKTR-118) with selected opioids, with the goal of treating pain without the side effect of constipation traditionally associated with opioid therapy.
Nektar and AstraZeneca have a global agreement for both naloxegol (NKTR-118) and NKTR-119. Under the agreement, AstraZeneca has responsibility for the development, global manufacturing and marketing of both naloxegol (NKTR-118) and NKTR-119. For naloxegol (NKTR-118), Nektar is eligible to receive up to $235 million in aggregate payments upon the achievement of certain regulatory milestones, as well as additional tiered sales milestone payments of up to $375 million if the product achieves considerable levels of commercial success. Nektar will also be eligible to receive significant double-digit royalty payments on net sales of naloxegol (NKTR-118) worldwide. For NKTR-119, Nektar would receive development milestone payments as well as tiered sales milestone payments. Nektar will also receive significant double-digit royalty payments on NKTR-119 net sales worldwide.
| The AstraZeneca Phase 3 KODIAC Program for Naloxegol (NKTR-118) The KODIAC Program consists of two randomized, placebo controlled Phase III efficacy studies and an open-label, randomized, placebo-controlled long term safety study. The two efficacy studies are identical with 12-week treatment periods. These studies are intended to evaluate the efficacy, safety and tolerability of an AstraZeneca investigational drug in patients with OIC. KODIAC is part of the KODIAC program of studies looking to determine whether naloxegol (NKTR-118) is safe and effective for the treatment of constipation seen as a side effect in people taking prescription opioid pain medications. AstraZeneca plans the first regulatory filings based on the program in 2013. |
References
http://newdrugapprovals.wordpress.com/2013/09/28/ema-accepts-astrazenecas-naloxegol-application/

DAGLUTRIL, SLV306
phase 2
Daglutril is a novel dual-action endopeptidase inhibitor which had been in phase II clinical development by Solvay for the treatment of hypertension, congestive heart failure (CHF) and pulmonary hypertension; however, no recent development has been reported for this research.
Daglutril inhibits NEP (neutral endopeptidase) and ECE (endothelin-converting enzyme) and thereby exerts vasodilating, blood pressure-lowering and other potentially beneficial effects on the cardiovascular system.
| 2-[3(S)-[1-[2(R)-(Ethoxycarbonyl)-4-phenylbutyl]cyclopentan-1-ylcarboxamido]-2-oxo-2,3,4,5-tetrahydro-1H-1-benzazepin-1-yl]acetic acid | |
| cas | 182821-27-8, 182560-84-5 (undefined stereochem.) |
| C31H38N2O6 | |
| mw | 534.6492 |
| Cardiovascular Drugs, Heart Failure Therapy, Hypertension, Treatment of, Endothelin-Converting Enzyme Inhibitors, Neprilysin Inhibitors |
SOLWAY
Neprilysin (Enkephalinase, Neutral Endopeptidase, NEP) Inhibitors
Endothelin-Converting Enzyme (ECE) Inhibitors
182821-27-8, 1H-1-Benzazepine-1-acetic acid, 3-(((1-(2-(ethoxycarbonyl)-4-phenylbutyl)cyclopentyl)carbonyl)amino)-2,3,4,5-tetrahydro-2-oxo-, (S-(R*,S*))-, 1H-1-Benzazepine-1-acetic acid, 3-(((1-((2R)-2-(ethoxycarbonyl)-4-phenylbutyl)cyclopentyl)carbonyl)amino)-2,3,4,5-tetrahydro-2-oxo-, (3S)-, 2-[(3S)-3-[[1-[(2R)-2-carbethoxy-4-phenyl-butyl]cyclopentanecarbonyl]amino]-2-keto-4,5-dihydro-3H-1-benzazepin-1-yl]acetic acid,
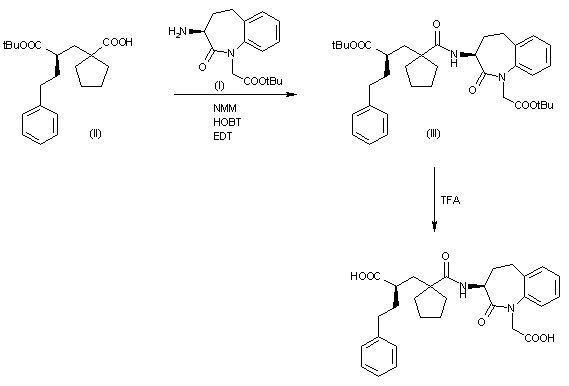
The acylation of the chiral amine (I) with the chiral cyclopentanecarboxylic aid (II) by means of N-methylmorphline (NMM), hydroxybenzotriazole (HOBT) and N-(dimethylaminopropyl)-N’-ethylcarbodiimide (EDT) in dichloromethane gives the amide (III), which is then treated with trifluoroacetic acid to elimnate the tert-butyl ester groups.
| Benzazepin-, benzoxazepin- and benzothiazepin-N-acetic acid-derivs., their preparation and their pharmaceutical compsns. | |
| Waldeck, H.; ET AL (Kali-Chemie AG) | |
| CA 2172354; EP 0733642; JP 1996269011; US 5677297 | |
 |
|
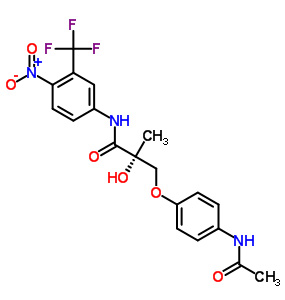

(SARM-4, S-4), GTx-007
Acetamidoxolutamide
Androxolutamide
401900-40-1
WO 2002016310
Selective Androgen Receptor Modulators (SARM)
Signal Transduction Modulators
Andarine (GTx-007, S-4) is an investigational selective androgen receptor modulator (SARM) developed by GTX, Inc for treatment of conditions such as muscle wasting, osteoporosis and benign prostatic hypertrophy, using the non-steroidal androgen antagonist bicalutamide as a lead compound.
Androxolutamide is a nonsteroidal selective androgen receptor modulator (SARM) which had been in early clinical trials at GTx for the treatment of cancer-related cachexia in several cancer types; however, no recent development has been reported for this indication. Preclinical studies had also been ongoing for the treatment of osteoporosis due to androgen deficiency in the aging male. The drug candidate is believed to bind to the testosterone receptor in such a way as to maximize the beneficial effects of the hormone like muscle growth, bone strengthening and enhanced libido, while minimizing the unwanted side effects, such as stimulation of prostate cancer, virilization and acne. This is accomplished by the selective modulation of the androgen receptor depending on tissue type.
The compound was originally developed at GTx. In March 2004, GTx entered into a joint collaboration and license agreement with Ortho Biotech, a wholly-owned subsidiary of Johnson & Johnson; however, in 2006 the agreement was terminated by mutual agreement of the companies.
Andarine is an orally active partial agonist for androgen receptors. It is less potent in both anabolic and androgenic effects than other SARMs. In an animal model of benign prostatic hypertrophy, andarine was shown to reduce prostate weight with similar efficacy to finasteride, but without producing any reduction in muscle mass or anti-androgenic side effects. This suggests that it is able to competitively block binding of dihydrotestosterone to its receptor targets in the prostate gland, but its partial agonist effects at androgen receptors prevent the side effects associated with the anti-androgenic drugs traditionally used for treatment of BPH
Family: Selective Androgen Receptor Modulator
Half Life: About 4 hours
Formula: C19 H18 F3 N3 O6
Chemical Structure: S-3-(4-acetylamino-phenoxy)-2-hydroxy-2-methyl-N-(4-nitro-3-trifluoromethyl-phenyl)-propionamide
Anabolic Rating: Similar to Testosterone Propionate
Facts: Ostarine (*S-4) is a Selective Androgen Receptor Modulator produced by GTx Inc, which is currently in the investigational stages of development. A SARM is exactly what it sounds like: a compound (not an anabolic steroid) which has the ability to stimulate the androgen receptor (much the same way as anabolic steroids). Unfortunately, due to its status as a drug still in the developmental stage, most of the research on it has been done in rodents and trials only.
S-4 is an orally active (and highly bioavailable) selective agonist for androgen receptors which was shown to have anabolic effects in muscle and bone tissue. It has been shown to have no measurable effect on lutenizing hormone (LH) or follicle-stimulating hormone (FSH), but it has been shown to have some effect on prostate weight, with an androgenic potency around 1/3rd of its anabolic potency (1). Still, this is a good trade-off, because it’s anabolic effect has been measured to be roughly the same as testosterone. It has also been shown to produce dose-dependent increases in bone mineral density and mechanical strength in addition to being able decrease body fat and increase lean body mass (2).
Unfortunately, it has a short half-life in humans of only 4 hours (3), and thus far has only gone through phase II clinical testing in humans (4).
Practical Use: This compound has potential use for all aspects of male hormone replacement therapy, and could eventually replace testosterone for this purpose. Since there is currently no accepted test for SARMs, athletes who are subject to drug testing would find it to be a suitable replacement for anabolic steroid use. Since it doesn’t effect LH or FSH, it may also be a highly useful anabolic agent to be used while attempting post-cycle therapy.
Side Effects: Prostate enlargement (1/3rd of what is seen with testosterone) and potential acne are potential side effects, although most users don’t report either of them; much more common are vision problems (floaters, yellow-tinged vision). Water retention, gynecomastia, and most other steroid-related side effects are probably not possible. In addition, inhibition of natural hormone levels is probably minimal or nonexistent at worst.
Producing/Developing Company:
Ostarine by GTx Inc.
References:

The androgen receptor (′AR′″) is a ligand-activated transcriptional regulatory protein that mediates induction of male sexual development and function through its activity with endogenous androgens. Androgens are generally known as the male sex hormones. However, androgens also play a pivotal role in female physiology and reproduction. The androgenic hormones are steroids which are produced in the body by the testis and the cortex of the adrenal gland, or synthesized in the laboratory. Androgenic steroids play an important role in many physiologic processes, including the development and maintenance of male sexual characteristics such as muscle and bone mass, prostate growth, spermatogenesis, and the male hair pattern (Matsumoto, Endocrinol. Met. Clin. N. Am. 23:857-75 (1994). The endogenous steroidal androgens include testosterone and dihydrotestosterone (“DHT”) Testosterone is the principal steroid secreted by the testes and is the primary circulatiag androgen found in the plasma of males. Testosterone is converted to DHT by the enzyme 5 alpha-reductase in many peripheral tissues. DHT is thus thought to serve as the intracellular mediator for most androgen actions (Zhou, et al., Molec. Endocrinol. 9:208-18 (1995)). Other steroidal androgens include esters of testosterone, such as the cypionate, propionate, phenylpropionate, cyclopentylpropionate, isocarporate, enanthate, and decanoate esters, and other synthetic androgens such as 7-Methyl-Nortestosterone (“MENT′”) and its acetate ester (Sundaram et al., “7 Alpha-Methyl-Nortestosterone(MENT): The Optimal Androgen For Male Contraception,” Ann. Med., 25:199-205 (1993) (“Sundaram”)). Because the AR is involved in male sexual development and function, the AR is a likely target for effecting male contraception or other forms of hormone replacement therapy. The AR also regulates female sexual function (i.e., libido), bone formation, and erythropoiesis.
Worldwide population growth and social awareness of family planning have stimulated a great deal of research in contraception. Contraception is a difficult subject under any circumstances. It is fraught with cultural and social stigma, religious implications, and, most certainly, significant health concerns. This situation is only exacerbated when the subject focuses on male contraception. Despite the availability of suitable contraceptive devices, historically, society has looked to women to be responsible for contraceptive decisions and their consequences. Although health concerns over sexually transmitted diseases have made men more aware of the need to develop safe and responsible sexual habits, women still often bear the brunt of contraceptive choice. Women have a number of choices, from temporary mechanical devices such as sponges and diaphragms to temporary chemical devices such as spermicides. Women also have at their disposal more permanent options, such as physical devices like IUDs and cervical caps as well as more permanent chemical treatments, such as birth control pills and subcutaneous implants. However, to date, the only options available for men include the use of condoms or a vasectomy. Condom use, however is not favored by many men because of the reduced sexual sensitivity, the interruption in sexual spontaneity, and the significant possibility of pregnancy caused by breakage or misuse. Vasectomies are also not favored If more convenient methods of birth control were available to men, particularly long term methods that require no preparative activity immediately prior to a sexual act, such methods could significantly increase the likelihood that men would take more responsibility for contraception.
Administration of the male sex steroids (e.g., testosterone and its derivatives) has shown particular promise in this regard due to the combined gonadotropin-suppressing and androgen-substituting properties of these compounds (Steinberger et al, “Effect of Chronic Administration of Testosterone Enanthate on Sperm Production and Plasma Testosterone, Follicle Stimulating Hormones and Luteinizing Hormone Levels: A Preliminary Evaluation of a Possible Male Contraceptive”, Fertility and Sterility 28:1320-28 (1977)). Chronic administration of high doses of testosterone completely abolishes sperm production (azoospermia) or reduces it to a very low level (oligospermia). The degree of spermatogenic suppression necessary to produce infertility is not precisely known, However, a recent report by the World Health Organization showed that weekly intramuscular injections of testosterone enanthate result in azoospermia or severe oligospermia (i.e., less than 3 million sperm per ml) and infertility in 98% of men receiving therapy (World Health Organization Task Force on Methods Ar Regulation of Male Fertility, “Contraceptive Efficacy of Testosterone-Induced Azoospermia and Oligospermia in Normal Men,” Fertilily and Sterility 65:821-29 (1996)).
A variety of testosterone esters have been developed that are more slowly absorbed after intramuscular injection ancd, thus, result in greater androgenic effect. Testosterone enanthate is the most widely used of these esters. While testosterone enanthate has been valuable in terms of establishing the feasibility of hormonal agents for male contraception, it has several drawbacks, including the need for weekly injections and the presence of supraphysiologic peak levels of testosterone immediately following intramuscular injection (Wu, “Effects of Testosterone Enanthate in Normal Men: Experience From a Multicenter Contraceptive Efficacy Study,” Fertility and Sterility 65:626-36 (1996)).
“male drugs”. D. D. Miller, K. A. Veverka, and K. Chung report the large-scale synthesis of androgen-receptor modulators exemplified by 3a and 3b. These compounds have a variety of pharmaceutical applications related to male sex hormones, such as male contraceptives and drugs for treating prostate-related conditions. The inventors describe the kilogram-scale production of 3a and 3b by condensing 1 with 2a or 2b, as shown in Figure 1.

The reaction is carried out in the presence of a substantial excess of Cs2CO3 in THF. For the preparation of 3a, 6.17 mol Cs2CO3 is used with 3.37 mol 1; for 3b, 5.4 mol Cs2CO3and 2.7 mol 1 are used. (Disconcertingly, the patent shows the formula of the base as CsCO3, although the calculation of the molar amount is correct.) The preparation of 3atakes 3 h at 50 °C and is monitored by HPLC. TLC is used to monitor the synthesis of3b, which takes 8 h in refluxing THF.
To purify 3a, deionized water is added to an EtOH solution at room temperature to precipitate it; this process is repeated three times. The final yield of 3a is 83%. Purifying the product by using an alcohol and water is a key aspect of the patent and is covered in the claims. However, no analytical data are given to support the claimed purity. The workup of 3b also involves EtOH and water, but solvents EtOAc and MeO-t-Bu are also used; the product is isolated in 52% yield.
The inventors also describe the synthesis of compound 1 at kilogram scale (Figure 2). Acid chloride 5 is prepared by the reaction of carboxylic acid 4 with SOCl2. The acid chloride is not isolated, but it is treated with a solution of aniline derivative 6 and Et3N in THF over 3 h. After it is warmed to room temperature, the mixture is heated to 50 °C for 15 h. The reaction is monitored by TLC; 3.7 kg 1 is isolated by crystallization from warm toluene in 70.3% yield.
The multikilogram-scale synthesis of 4 is also described. The route, shown in Figure 3, starts with the preparation of compound 9 by simultaneously adding 4 M NaOH and a solution of acid chloride 8 in acetone to a mixture of carboxylic acid 7 and 4 M NaOH in acetone. The pH of the reaction mixture is kept at >10 by adding more 4 M NaOH as needed. Intermediate 9 is isolated by crystallization from MeO-t-Bu in 55.6% yield; it is then treated with N-bromosuccinimide (NBS) in DMF to cyclize it to 10. This is isolated in 87.7% yield by adding water to the reaction mixture. The final step is heating 10 to reflux in 24% aq HBr to produce 4, isolated as a crystalline solid from hot toluene in 81.3% yield.
The patent claims cover compounds related to 3a and 3b in which the nitro group is replaced by nitrile. Unfortunately, no examples are given describing the synthesis of these compounds. This is an efficient process for synthesizing 3a and 3b, and the inventors show that it is suitable for large-scale production. (University of Tennessee Research Foundation [Knoxville]. US Patent 7,968,721, June 28, 2011;

Novel pathway for the synthesis of arylpropionamide-derived selective androgen receptor modulator (SARM) metabolites of andarine and ostarine
TETRAHEDRON LETTERS,
Pages 2239-2242
Katharina M. Schragl, Guro Forsdahl, Guenter Gmeiner, Valentin S. Enev, Peter Gaertner
andexanet alfa

Portola gets FDA breakthrough therapy status for andexanet alfa
US-based biopharmaceutical firm Portola Pharmaceuticals has received breakthrough therapy designation from the US Food and Drug Administration (FDA) for its investigational Factor Xa inhibitor antidote, ‘andexanet alfa’.
read all at
Description
Key Characteristics
Potential Indications
Clinical Development
Phase 2 proof-of-concept studies are underway or planned. These randomized, double-blind, placebo-controlled studies are designed to assess the safety, tolerability, pharmacokinetics and pharmacodynamics of andexanet alfa after dosing of a direct/indirect Factor Xa inhibitor in healthy volunteers.
 |
|
|---|---|
 |
|
| Combination of | |
| Tetrahydrocannabinol | Cannabinoid |
| Cannabidiol | Cannabinoid |
GW Pharmaceuticals obtains Swiss approval for Sativex
GW Pharmaceuticals has received full marketing authorisation from the Swiss authorities for its prescription medicine Sativex to treat moderate to severe spasticity in multiple sclerosis (MS) patients who have not responded to other medications.
Nabiximols (USAN,trade name Sativex) is a patented cannabinoid oromucosal mouth spray developed by the UK company GW Pharmaceuticals for multiple sclerosis (MS) patients, who can use it to alleviate neuropathic pain, spasticity, overactive bladder, and other symptoms.Nabiximols is distinct from all other pharmaceutically produced cannabinoids currently available because it is a mixture of compounds derived fromCannabis plants, rather than a mono-molecular synthetic product. The drug is a pharmaceutical product standardised in composition, formulation, and dose, although it is still effectively a tincture of the cannabis plant. Its principal active cannabinoid components are the cannabinoids: tetrahydrocannabinol (THC) and cannabidiol (CBD). The product is formulated as an oromucosal spray which is administered by spraying into the mouth. Each spray delivers a near 1:1 ratio of CBD to THC, with a fixed dose of 2.7 mg THC and 2.5 mg CBD. Nabiximols is also being developed in Phase III trials as a potential treatment to alleviate pain due to cancer. It has also been researched in various models of peripheral and central neuropathic pain.
In May 2003 GW Pharmaceuticals and Bayer entered into an exclusive marketing agreement for GW’s cannabis-based medicinal extract product, to be marketed under the brand name Sativex. “Bayer has obtained exclusive rights to market Sativex in the UK. In addition, Bayer has the option for a limited period of time to negotiate the marketing rights in other countries in European Union and selected other countries around the world.”
In April 2011, GW licensed to Novartis the rights to commercialise nabiximols in Asia (excluding China and Japan), Africa and the Middle East (excluding Israel)
Of the two preliminary Phase III studies investigating the treatment of MS patients, one showed a reduction of spasticity of 1.2 points on the 0–10 points rating scale (versus 0.6 points under placebo), the other showed a reduction of 1.0 versus 0.8 points. Only the first study reached statistical significance. The Phase III approval study consisted of a run-in phase where the response of individuals to the drug was determined. The responders (42% of patients) showed a significant effect in the second, placebo controlled, phase of the trial.[10] A 2009 meta-analysis of six studies found large variations of effectiveness, with a trend towards a reduction of spasticity
Sativex® has now been launched in 11 countries (including the UK, Spain, Italy and Germany) with approvals in an additional 11 countries

PRANLUKAST
Antiasthmatic.
| Launched – 1995 japan150821-03-7, C27 H23 N5 O4 . H2O, 499.5179 |
103177-37-3 anhydrous, 103180-28-5 (monosodium salt)
Ono-1078
Ono-RS-411
RS-411
SB-205312
Ono-1070 (monosodium salt)
N-[4-Oxo-2-(1H-tetrazol-5-yl)-4H-1-benzopyran-8-yl]-4-(4-phenylbutoxy)benzamide hemihydrate
Ono (Originator)Schering-Plough (Licensee)
……….
J Med Chem 1988, 31(1): 84,
WO 2010002075,
Synth Commun 1997, 27(6): 1065,
WO 1994012492
Leukotriene antagonist.
Prepn: M. Toda et al., EP 173516; eidem, US 4780469 (1986, 1988 both to Ono);
H. Nakai et al., J. Med. Chem. 31, 84 (1988).
Pharmacology: T. Obata et al., Adv. Prostaglandin Thromboxane Leukotriene Res. 15, 229 (1985); idem et al., ibid. 17, 540 (1987).
Clinical evaluations in asthma: Y. Taniguchi et al., J. Allergy Clin. Immunol. 92, 507 (1993); H. Yamamoto et al. Am. J. Respir. Crit. Care Med. 150, 254 (1994).
AU 8546462; EP 0173516; JP 8650977; US 4780469; US 4939141
Pranlukast is a cysteinyl leukotriene receptor-1 antagonist. It antagonizes or reduces bronchospasm caused, principally in asthmatics, by an allergic reaction to accidentally or inadvertently encountered allergens.
Pranlukast is a cysteinyl leukotriene receptor-1 antagonist. This drug works similarly to Merck & Co.‘s Singulair (montelukast). It is widely used in Japan.
Medications of this class, which go under a variety of names according to whether one looks at the American, British or European system of nomenclature, have as their primary function the antagonism of bronchospasm caused, principally in asthmatics, by an allergic reaction to accidentally or inadvertently encountered allergens.
Medications of this group are normally used as an adjunct to the standard therapy of inhaled steroids with inhaled long- and/or short-acting beta-agonists. There are several similar medications in the group; all appear to be equally effective.
Toda synthetic complete with 3 – nitro-2 – hydroxyphenyl ko one for raw materials, ni ko with oxalic ester Claisen condensation occurs, and then heated to reflux for cyclization to construct benzo pyran ring; dehydrated by an amide synthesized ring cyano group, the cyano compound and then with sodium azide tetrazole synthesis. The nitro group on the compound in 5% Pd / C catalyzed hydrogenation of amino acid reacted with the compound Pranlukast held. This method directly using 4 – (4 – phenyl-butoxy)-benzoic acid reaction. Synthetic route is as follows:
[0006]
[0007]
[0008] ② Robert Graham and routes are routes to I-bromo-butane as a raw material, were used as a palladium catalyst, ligand compound formylation carbonylation reactions and condensation of potassium tert-butoxide, closed dehydration under acidic conditions benzopyran ring method. Synthetic route is as follows:
[0009] Robert routes:
[0010]
[0011] Graham route:
[0012]
[0013] The two synthetic routes are not disclosed in the I-Bromo butane feedstock pathway.
[0014] ③ Masayohi 2_ cyano synthetic route to a benzopyran derivative and hydrogen sulfide gas in the base-catalyzed addition reaction of 2 – thiocarbamoylbenzothiazol and pyran derivatives, and then were reacted with anhydrous hydrazine group hydrazone, with sodium nitrite under acidic conditions nitrosation reaction occurs tetrazole ring. Synthetic route is as follows:
[0015]
[0016] The materials used are not mentioned route synthesis method, it is only reflected in the improvement of the synthesis of the tetrazole ring.
[0017] ④ Giles, Hideki and Hayler are tetrazole substituent on the increase, making it easier condensation reaction, but the synthesis of substituted on the nitrogen with tetrazole difficult, and ultimately elimination reaction of lithium used tetrahydro aluminum and other hazardous reagents, is not easy to Eri industrialization. Reaction scheme is as follows:
[0018]
[0019] ⑤ Lee NK with 4_ (4_ Phenylbutoxy) benzonitrile and 2_ hydroxy _3_ iodobenzene ko 1H_4_ thiazolyl ketone and ester ko _5_ acid, concentrated sulfuric acid catalyzed cyclization iodide copper and potassium phosphate removal under the action of hydrogen iodide get Pranlukast held. Reaction scheme is as follows:
[0021] does not mention the route starting 4 – (4 – phenyl-butoxy)-benzonitrile synthesis method, while two – hydroxy – 3 – Synthesis of iodobenzene ko difficult one.

The synthesis method comprises the following steps: a. 4 – Synthesis of chlorobutanol THF was added concentrated hydrochloric acid, feeding the mass ratio of I: I. 389 ~ 5. 556,45-80 ° C was stirred for 5-18h, cooled, extracted with methylene chloride, removal of the solvent, distillation under reduced pressure to give 4 – chlorobutanol; b. 4 – phenyl butanol take benzene, aluminum chloride mixture ,0-25 ° C solution of 4 – chlorobutanol, reaction 5 -10h then poured into ice-water, a liquid, in addition to homogeneous solution U, distillation under reduced pressure, and the resulting colorless transparent liquid that is, 4 – phenyl butanol; c. I-bromo-4 – phenyl butane synthesis of 4 – phenyl butanol 40% hydrobromic acid mixture, feeding the mass ratio of I: 2. 857 ~ 11. 428, heat refluxing, cooling, liquid separation, the organic solvent divided by distillation under reduced pressure to give I-bromo-4 – phenyl butane; d. Synthesis of methyl p-hydroxybenzoate take-hydroxybenzoic acid and methanol, concentrated sulfuric acid and refluxed for 5-20h spin methanol, poured into cold water to precipitate a white solid which was filtered and dried to give the hydroxy benzoate; e. 4 – (4 – phenyl-butoxy)-benzoic acid methyl ester _ take I-bromo-4 – phenyl butane, DMF, toluene, methyl p-hydroxybenzoate and potassium carbonate, a reflux 5 ~ 20h, cooling water, extracted with toluene, light yellow liquid rotary evaporation, recrystallization, and the resulting white solid, that is, 4 – (4 – phenyl-butoxy) – benzoic acid methyl ester; f. 4 – (4 – phenyl-butoxy yl) – benzoic acid taken 4 – (4 – phenyl-butoxy) – benzoic acid methyl ester, 15% NaOH solution was refluxed for I ~ 5h, cooled, acidified, filtered and dried to give 4 – (4 – phenylbutyrate oxy) – benzoic acid; g. sprinkle bromophenyl acetic acid ester molar ratio Preparation of I: I ~ I. 5: O. I ~ I of bromophenol, acetic anhydride, pyridine feeding, reflux 3 ~ 10h, distilled pyridine, acetic acid and excess acetic anhydride distilled under reduced pressure to give the acetic acid esters bromophenol; h. 5 – bromo-2 – Preparation of light taken acetophenone molar ratio of I: I ~ 5: I of acetic acid bromophenol esters, aluminum chloride, tetrachlorethylene for feeding, reflux O. 5 ~ 5. 5h, cooled, the reaction solution was poured into 5% hydrochloric acid and extracted with methylene chloride, the solvent evaporated under reduced pressure, to obtain a gray crystalline 5 – bromo-2 – Light acetophenone; i. 5 – bromo-3 – nitro-2 – Preparation of light acetophenone take 5 – bromo-2 – Light acetophenone, carbon tetrachloride, 50 ~ 90 ° C is added dropwise nitric acid, reflux I ~ 4h, cooled, filtered, and the resulting yellow solid which is 5 – bromo-3 – nitro-2 – hydroxyacetophenone; j. 3 – amino-2 – Light benzene ethanone Preparation of 5 – bromo-3 – nitro-2 – hydroxyacetophenone, 5% Pd / C, methylene chloride, methanol, concentrated hydrochloric acid, water, hydrogenation; the end of the reaction mixture was filtered, the filtrate was The solvent was removed, neutralized with sodium bicarbonate, and the resulting yellow solid ginger i.e., 3 – amino-2 – hydroxyacetophenone; k. 3 – [4 - (4 - phenyl-butoxy)-benzoyl amino] -2 _ light base Preparation of acetophenone 4 – (4 – phenyl-butoxy)-benzoic acid, toluene, DMF, 45 ~ 105 ° C was added dropwise SOCl2, 30min the reaction liquid droplets to the 3 – amino-2 – hydroxyphenyl toluene solution of ethyl ketone, the reaction 3 ~ 10h, cooled, neutralized with dilute hydrochloric acid, extracted with toluene, rotary evaporation, and the resulting pale yellow crystals is 3 – [4 - (4_ phenylbutoxy) benzamido] 2_-hydroxyacetophenone; I. 2 – [4 - (4 - phenyl-butoxy)-benzoyl amino] -6 – [l, 3 - dioxo-3 - ethoxycarbonyl-propyl] phenol synthetic sodium, THF, 3 – [4 - (4 - phenyl-butoxy)-benzoyl amino]-2 – hydroxyacetophenone, diethyl oxalate 4 ~ IOh After stirring the reaction was poured into dilute hydrochloric acid to precipitate the yellow solid which was filtered, and the resulting product, i.e. 2 – [4 - (4_ phenylbutoxy) benzamido] _6_ [1,3 - dioxo-3 - ethoxy propyl intended yl] phenyl discretion ·; m. 4 – oxo-8 – [4 - (4 - phenyl-butoxy)-benzoyl amino]-2 – ethoxycarbonyl-4H-benzopyran take 2 – [4 - (4 - phenyl-butoxy yl) benzoyl amino] -6 – [l, 3 - dioxo-3 - ethoxycarbonyl-propyl] phenol, THF, force mouth heat, the addition of concentrated hydrochloric acid, refluxed for 8 ~ 15h, cooled, filtered, and the resulting white solid, that is, 4 – oxo-8 – [4 - (4 - phenyl-butoxy)-benzoyl amino]-2 – ethoxycarbonyl-4H-benzopyran; η. 4 – oxo-8 – [ 4 - (4 - phenyl-butoxy)-benzoyl amino] -2 – amino-carbonyl-4Η-benzopyran synthesis take four – oxo-8 – [4 - (4 - phenyl-butoxy)-benzoyl amino] -2 – ethoxycarbonyl-4Η-benzopyran was dissolved in DMF, and leads to dry ammonia gas, the reaction solution changed from yellow to red, the reaction solution was poured into cold water, adjusted to acidic, and filtered to give the product 4 – oxo-8 – [4 - (4 - phenyl-butoxy)-benzoyl amino] -2 – amino-carbonyl-4Η-benzopyran; P. 4 – oxo-8 – [4 - (4 - phenylbutoxy) benzamido] -2 – cyano-4Η-benzopyran take DMF, S0C12, 4 – oxo-8 – [4 - (4 - phenyl-butoxy)-benzoic amido] _2_ aminocarbonyl-4H-benzopyran, O ~ 15 ° C under stirring for 2 ~ IOh poured into cold water, filtered, and the resulting white solid that is, 4 – oxo-8 – [4 - (4 - phenylbutoxy) benzamido] -2 – cyano-4H-benzopyran; q. Synthesis of pranlukast take four – oxo-8 – [4 - (4 - phenyl-butoxy) benzoyl amino]-2_ cyano-4H-benzopyran, ammonium chloride, sodium azide, DMF, heating I ~ 8h then poured into ice-water, dilute hydrochloric acid, filtered, and the resulting white solid that the final product Pranlukast.

The reaction of ethyl 8-nitro-4-oxo-1-benzopyran-2-carboxylate (I) with ammonia in methanol gives the corresponding amide (II), which is dehydrated with POCl3 yielding 2-cyano-8-nitro-1-benzopyran-4-one (III). The cyclization of (III) with sodium azide by means of pyridinium chloride in hot DMF affords 8-nitro-2-(tetrazol-5-yl)-1-benzopyran-4-one (IV), which is hydrogenated with H2 over Pd/C in methanol – HCl giving 8-amino-2-(tetrazol-5-yl)-1-benzopyran-4-one (V). Finally, this compound is condensed with 4-(4-phenylbutoxy)benzoic acid (VI) by means of oxalyl chloride in dichloromethane-pyridine

254750-02-2 cas no
emricasan
PF 03491390, IDN 6556
pfizer
Prevention of fibrosis and inflammation in chronic liver disease
The compound had been studied in phase II clinical trials for the treatment of liver transplant rejection and hepatitis B
(3S)-3-[[(2S)-2-[[2-[(2-tert-butylphenyl)amino]-2-oxoacetyl]amino]propanoyl]amino]-4-oxo-5-(2,3,5,6-tetrafluorophenoxy)pentanoic acid, C26 H27 F4 N3 O7, 569.5
http://www.ama-assn.org/resources/doc/usan/emricasan.pdf
Conatus’s liver drug emricasan gets FDA orphan drug status
US-based biotechnology firm Conatus Pharmaceuticals has received orphan drug designation from the US Food and Drug Administration (FDA) for its drug candidate emricasan to treat liver transplant recipients with re-established fibrosis to delay the progression to cirrhosis and end-stage liver disease.http://www.pharmaceutical-technology.com/news/newsconatuss-chronic-liver-disease-treatment-emricasan-gets-fda-orphan-drug-status-4139697?WT.mc_id=DN_News
Emricasan, also known as IDN 6556 and PF 03491390, is a first-in-class caspase inhibitor in clinical trials for the treatment of liver diseases. IDN-6556 has marked efficacy in models of liver disease after oral administration and thus, is an excellent candidate for the treatment of liver diseases characterized by excessive apoptosis. IDN-6556 appears to be a feasible therapeutic agent against ischemia-reperfusion injury in liver transplantation.
WO 2002057298
WO 2000001666
Interleukin 1 (“IL-1″) is a major pro-inflammatory and immunoregulatory protein that stimulates fibroblast differentiation and proliferation, the production of prostaglandins, collagenase and phospholipase by synovial cells and chondrocytes, basophil and eosinophil degranulation and neutrophil activation. Oppenheim, J.H. et al.. Immunology Today, 7:45-56 (1986). As such, it is involved in the pathogenesis of chronic and acute inflammatory and autoimmune diseases. IL-1 is predominantly produced by peripheral blood monocytes as part of the inflammatory response. Mosely, B.S. et al.. Proc. Nat. Acad. Sci.. 84:4572-4576 (1987); Lonnemann, G. et al. Eur. J. Immunol., 19:1531-1536 (1989).
IL-lβ is synthesized as a biologically inactive precursor, proIL-lβ. ProIL-lβ is cleaved by a cysteine protease called interleukin-lβ converting enzyme (“ICE”) between Asp-116 and Ala-117 to produce the biologically active C-terminal fragment found in human serum and synovial fluid. Sleath, P.R. et al., J. Biol. Chem., 265:14526-14528 (1992); A.D. Howard et al, J. Immunol., 147:2964-2969 (1991).
ICE is a cysteine protease localized primarily in monocytes. In addition to promoting the pro -inflammatory and immunoregulatory properties of IL-lβ, ICE, and particularly its homologues, also appear to be involved in the regulation of cell death or apoptosis. Yuan, J. et al„ Cell, 75:641-652 (1993); Miura, M. et al. Cell, 75:653-660 (1993); Nett-Giordalisi, M.A. et al, J. Cell Biochem., 17B:117 (1993). In particular, ICE or ICE/ced-3 homologues are thought to be associated with the regulation of apoptosis in neurogenerative diseases, such as Alzheimer’s and Parkinson’s disease. Marx, J. and M. Baringa, Science, 259:760-762 (1993); Gagliardini, N et al„ Science, 263:826-828 (1994).
Thus, disease states in which inhibitors of the ICE/ced-3 family of cysteine proteases may be useful as therapeutic agents include: infectious diseases, such as meningitis and salpingitis; septic shock, respiratory diseases; inflammatory conditions, such as arthritis, cholangitis, colitis, encephalitis, endocerolitis, hepatitis, pancreatitis and reperfusion injury, ischemic diseases such as the myocardial infarction, stroke and ischemic kidney disease; immune-based diseases, such as hypersensitivity; auto-immune diseases, such as multiple sclerosis; bone diseases; and certain neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease. Such inhibitors are also useful for the repopulation of hematopoietic cells following chemo- and radiation therapy and for prolonging organ viability for use in transplantation.
ICE/ced-3 inhibitors represent a class of compounds useful for the control of the above-listed disease states. Peptide and peptidyl inhibitors of ICE have been described. However, such inhibitors have been typically characterized by undesirable pharmacologic properties, such as poor oral absorption, poor stability and rapid metabolism. Plattner, J.J. and D.W. Norbeck, in Drug Discovery Technologies, C.R. Clark and W.H. Moos, Eds. (Ellis Horwood, Chichester, England, 1990), pp. 92-126. These undesirable properties have hampered their development into effective drugs.
Accordingly, the need exists for compounds that can effectively inhibit the action of the ICE/ced-3 family of proteases, for use as agents for preventing unwanted apoptosis, and for treating chronic and acute forms of IL-1 mediated diseases such as inflammatory, autoimmune or neurodegenerative diseases. The present invention satisfies this need and provides further related advantages.
|
References |
1: McCall M, Toso C, Emamaullee J, Pawlick R, Edgar R, Davis J, Maciver A, Kin T, Arch R, Shapiro AM. The caspase inhibitor IDN-6556 (PF3491390) improves marginal mass engraftment after islet transplantation in mice. Surgery. 2011 Jul;150(1):48-55. doi: 10.1016/j.surg.2011.02.023. Epub 2011 May 18. PubMed PMID: 21596412.
2: Pockros PJ, Schiff ER, Shiffman ML, McHutchison JG, Gish RG, Afdhal NH, Makhviladze M, Huyghe M, Hecht D, Oltersdorf T, Shapiro DA. Oral IDN-6556, an antiapoptotic caspase inhibitor, may lower aminotransferase activity in patients with chronic hepatitis C. Hepatology. 2007 Aug;46(2):324-9. PubMed PMID: 17654603.
3: Hoglen NC, Anselmo DM, Katori M, Kaldas M, Shen XD, Valentino KL, Lassman C, Busuttil RW, Kupiec-Weglinski JW, Farmer DG. A caspase inhibitor, IDN-6556, ameliorates early hepatic injury in an ex vivo rat model of warm and cold ischemia. Liver Transpl. 2007 Mar;13(3):361-6. PubMed PMID: 17318854.
4: Baskin-Bey ES, Washburn K, Feng S, Oltersdorf T, Shapiro D, Huyghe M, Burgart L, Garrity-Park M, van Vilsteren FG, Oliver LK, Rosen CB, Gores GJ. Clinical Trial of the Pan-Caspase Inhibitor, IDN-6556, in Human Liver Preservation Injury. Am J Transplant. 2007 Jan;7(1):218-25. PubMed PMID: 17227570.
5: Poordad FF. IDN-6556 Idun Pharmaceuticals Inc. Curr Opin Investig Drugs. 2004 Nov;5(11):1198-204. Review. PubMed PMID: 15573871.
6: Hoglen NC, Chen LS, Fisher CD, Hirakawa BP, Groessl T, Contreras PC. Characterization of IDN-6556 (3-[2-(2-tert-butyl-phenylaminooxalyl)-amino]-propionylamino]-4-oxo-5-(2,3,5,6-te trafluoro-phenoxy)-pentanoic acid): a liver-targeted caspase inhibitor. J Pharmacol Exp Ther. 2004 May;309(2):634-40. Epub 2004 Jan 23. PubMed PMID: 14742742.
7: Valentino KL, Gutierrez M, Sanchez R, Winship MJ, Shapiro DA. First clinical trial of a novel caspase inhibitor: anti-apoptotic caspase inhibitor, IDN-6556, improves liver enzymes. Int J Clin Pharmacol Ther. 2003 Oct;41(10):441-9. PubMed PMID: 14703949.
8: Canbay A, Feldstein A, Baskin-Bey E, Bronk SF, Gores GJ. The caspase inhibitor IDN-6556 attenuates hepatic injury and fibrosis in the bile duct ligated mouse. J Pharmacol Exp Ther. 2004 Mar;308(3):1191-6. Epub 2003 Nov 14. PubMed PMID: 14617689.
9: Natori S, Higuchi H, Contreras P, Gores GJ. The caspase inhibitor IDN-6556 prevents caspase activation and apoptosis in sinusoidal endothelial cells during liver preservation injury. Liver Transpl. 2003 Mar;9(3):278-84. PubMed PMID: 12619025.
//////////////////////////////////////////////////////////
http://www.google.com/patents/WO2002057298A2
EXAMPLE 126
(3 S)-3 – [N-(N'-(2-TERT-BUTYLPHENYL)OXAMYL) ALANINYL] AMINO-5-(2′,3′,5′,6′-TETRAFLUOROPHENOXY)-4-OXOPENTANOIC ACID
Part A: [(N-Benzyloxycarbonyl Alaninyl]Aspartic Acid, β-tert-Butyl Ester
To a suspension of aspartic acid β-tert-butyl ester (3.784 g, 20 mmol) in dimethylformamide (150 mL) at room temperture under nitrogen was added bis(trimethylsilyl)-trifluoroacetamide (10.6 mL, 40 mmol). After stirring at room temperature for 30 min, the resulting clear solution was treated with (N- benzyloxycarbonyl)alanine N-hydroxysuccinimide ester (6.406 g, 20 mmol). After stirring at room temperature for an additional 48 hrs, the mixture was treated with water (20 mL), stirred for 15 min and then partitioned between EtO Ac/water. The organic phase was washed with water, 5% KHSO and saturated NaCl solutions, dried over anhydrous Na2SO and evaporated to a dryness. The residue was dissolved in Et2O and extracted with saturated NaHCO3. The aqueous extract was acidified (pH 2.0) with concentrated HCl and extracted with EtOAc. The EtOAc extract was washed with saturated NaCl solution, dried over anhydrous Na2SO4 and evaporated to a give the title compound (6.463 g, 82%) as a white foam. TLC(EtOAc-hexane-AcOH; 70:30:2) Rf = 0.50.
Part B: (3S,4RS -3-rAlaninynAmino-5-(2′.3′.5′.6′-TetrafluorophenoxyV4- Hydroxypentanoic Acid tert-Butyl Ester
Starting with [(N-benzyloxycarbonyl)alanmyl]aspartic acid, β-tert-butyl ester and following the methods described in Example 28, Parts B through E gave the title compound as a colorless, viscous oil. TLC(EtOAc-hexane; 1:1) Rf = 0.06.
Part C: (3 S,4RS -3-[ -(Η'-f2-tert-Butylρhenyl)Oxamyl) AlaninyllAmino-5- (2',3',5',6'-Tetrafluorophenoxy)-4-Hvdroxypentanoic Acid tert-Butyl
Ester
To a solution of N-(2-tert-butylphenyl)oxamic acid (0.041 g, 0.19 mmol, prepared from 2-tert-butylaniline by the method described in Example 1, Part A) in
CH C1 (6.0 mL) at 0°C under nitrogen was added hydroxybenzofriazole hydrate (0.030 g) followed by l-ethyl-3 -(3 ',3 '-dimethyl- l'-aminopropyl)- carbodiimide hydrochloride
(0.050 g, 0.26 mmol). After stirring at 0°C for 10 min, the mixture was treated with
(3S,4RS)-3-(alaninyl)amino-5-(2',3',5',6'-tetrafluorophenoxy)-4-hydroxypentanoic acid tert-butyl ester (0.079 g, 0.19 mmol) and N-methylmorpholine (22 μL, 0.20 mmol).
After stirring at room temperature for 16 hrs, the mixture was partitioned between EtOAc-water. The organic phase was washed with water, 5% KHSO , saturated
NaHCO3 and saturated NaCl solutions, dried over anhydrous Na2SO4 and evaporated to give the crude title compound (0.090 g, 77%) as a viscous oil. TLC(EtOAc-hexane;
1:1) Rf= 0.70.
Part D: r3S -3-rN-rN'-(2-tert-Butylphenyl Oxamyl)AlaninyllAmino-5- (2',3',5'.6'-Tetrafluorophenoxy)-4-Oxopentanoic Acid tert-Butyl Ester
To a solution of (3S,4RS)-3-[N-(N'-(2-tert-butylphenyl)oxamyl)alaninyl] amino-5-(2′,3′,5′36′-tetrafluorophenoxy)-4-hydroxypentanoic acid tert-butyl ester (0.0.092 g, ca 0.15 mmol) in CH2C1 (6.5 mL) at room temperature under nitrogen was added iodobenzene diacetate (0.188 g, 0.58 mmol) followed by a catalytic amount of 2,2,6,6-tetramethyl-l-piperidinyloxy free radical (TEMPO, 0.0046 g, 0.03 mmol). After stirring at room temperature for 16 hrs, the mixture was partitioned between EtOAc- water. The organic phase was washed with saturated NaHCO3 and saturated NaCl solutions, dried over anhydrous Na SO4 and evaporated to a dryness. The residue (0.096 g) was purified by preparative layer chromatography on silica gel eluting with EtOAc- hexane (3:7) to give the title compound (0.071 g, 77%) as a colorless glass. TLC(EtOAc-hexane; 2:3) Rf = 0.60.
Part E: (3S)-3-rN-(N’-r2-tert-Butylphenyl Oxamyl Alaninyl]Amino-5- (2′ ,3 ‘ , 5 ‘ ,6′ -Tetrafluorophenoxy)-4-Oxopentanoic Acid
To a solution of (3S)-3-[N-(N'-(2-tert- butylphenyl)oxamyl)alaninyl]amino-5-(2′,3′,5′,6′-tetrafluorophenoxy)-4-oxopentanoic acid, tert-butyl ester (0.071 g, 0.11 mmol) in CH2C12(2.5 mL)-anisole(0.05 mL) at room temperature under nitrogen was added trifluoroacetic acid (1.5 mL). The resulting clear solution was stirred at room temperature for 1 hr, evaporated to dryness and chased with toluene-CH2Cl2 (1:1). The residue (0.061 g) was purified by preparative layer chromatography on silica gel eluting with MeOH-CH2Cl2 (1:9) to give the title compound (0.044 g, 69%) as a colorless glass. MS(ES) for C26H27F4N3O7 (MW 569.51): positive 570(M+H); negative 568(M-H).

TRANDOLAPRIL
(2S,3aR,7aS)-1-[(2S)-2-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl]-octahydro-1H-indole-2-carboxylic acid
87679-37-6 CAS NO
Indications. hypertention
Abbott..(opten , godrik, mavik), HOECHST MARION ROUSSEL..Odrik,
RU-44570, Preran,
Aventis Pharma (Originator), Nippon Roussel (Originator), Abbott (Licensee), Chugai (Licensee) Launched-1993
Launched-1993
Trandolapril is a non-sulhydryl prodrug that belongs to the angiotensin-converting enzyme (ACE) inhibitor class of medications. It is metabolized to its biologically active diacid form, trandolaprilat, in the liver. Trandolaprilat inhibits ACE, the enzyme responsible for the conversion of angiotensin I (ATI) to angiotensin II (ATII). ATII regulates blood pressure and is a key component of the renin-angiotensin-aldosterone system (RAAS). Trandolapril may be used to treat mild to moderate hypertension, to improve survival following myocardial infarction in clinically stable patients with left ventricular dysfunction, as an adjunct treatment for congestive heart failure, and to slow the rate of progression of renal disease in hypertensive individuals with diabetes mellitus and microalbuminuria or overt nephropathy.
Trandolapril is an ACE inhibitor used to treat high blood pressure, it may also be used to treat other conditions. It is marketed by Abbott Laboratories with the brand name Mavik.
Tarka is the brand name of an oral antihypertensive medication that combines a slow release formulation of verapamil hydrochloride, acalcium channel blocker, and an immediate release formulation of trandolapril, an ACE inhibtor. The patent, held by Abbott Laboratories, expires on February 24, 2015.
This combination medication contains angiotensin-converting enzyme (ACE) inhibitor and calcium channel blocker, prescribed for high blood pressure.

Trandolapril is a prodrug that is deesterified to trandolaprilat. It is believed to exert its antihypertensive effect through the renin-angiotensin-aldosterone system. Trandolapril has a half life of about 6 hours, and trandolaprilat has a half life of about 10. Trandolaprilat has about 8 times the activity of its parent drug. Approximately 1/3 of Trandolapril and its metabolites are excreted in the urine, and about 2/3 of trandolapril and its metabolites are excreted in the feces. Serum protein binding of trandolapril is about 80%.
Trandolapril is a drug that is used to lower blood pressure. Blood pressure is dependent on the degree of constriction (narrowing) of the arteries and veins. The narrower the arteries and veins, the higher the blood pressure. Angiotensin Il is a chemical substance made in the body that causes the muscles in the walls of arteries and veins to contract, narrowing the arteries and veins and thereby elevating blood pressure. Angiotensin Il is formed by an enzyme called angiotensin converting enzyme (ACE). Trandolapril is an inhibitor of ACE and blocks the formation of angiotensin Il thereby lowering blood pressure. The drop in blood pressure also means that the heart does not have to work as hard because the pressure it must pump blood against is less. The efficiency of a failing heart improves, and the output of blood from the heart increases. Thus, ACE inhibitors such as trandolapril are useful in treating heart failure.
Trandolapril‘s ACE-inhibiting activity is primarily due to its diacid metabolite, trandolaprilat, which is approximately eight times more active as an inhibitor of ACE activity.
……………………
synthesis

(3aR,7aS)-octahydroindole-2(S)-carboxylic acid (I) goes through the process of esterification with benzyl alcohol (II) in the presence of SOCl2 to produce the corresponding benzyl ester (III), and the yielding compound is then condensed with N-[1(S)-(ethoxycarbonyl)-3-phenylpropyl]-(S)-alanine (IV) in the presence of 1-hydroxybenzotriazole, N-ethylmorpholine and dicyclohexylcarbodiimide (DCC) in DMF to afford the benzyl ester (V) of the desired product. Lastly, the compound is debenzylated by hydrogenation with H2 over Pd/C in ethanol.
……………………………………………..
Trandolapril along with other related compounds was first disclosed in US4933361. The process for the synthesis of trandolapril was described in US4933361 and WO9633984.
US4933361 describes a process for the synthesis of trandolapril wherein the racemic benzyl ester of octahydro indole-2-carboxylic acid is reacted with N-[1-(S)-ethoxy carbonyl- 3- phenyl propyl]-L-alanine (ECPPA), to get racemic benzyl trandolapril, which is purified using column chromatography to get the 2S isomer of benzyl trandolapril, which is further debenzylated with Pd on carbon to get trandolapril as a foamy solid. This process has certain disadvantages, for example the product is obtained in very low yield. Purification is done using column chromatography, which is not suitable for industrial scale up.
WO9633984 discloses a process in which N-[1-(S)-ethoxy carbonyl-3- phenyl propyl]-L- alanine is activated with N-chlorosulfinyl imidazole, to get (N-[I-(S) N-[1-(S)-ethoxy carbonyl-3-phenyl propylj-L-alanyl-N-sulfonyl anhydride and which is further reacted with silyl-protected 2S,3aR,7aS octahydro indole 2-carboxyIic acid to obtain trandolapril. The main disadvantages of this process are that the silyl-protected intermediates are very sensitive to moisture, the process requires anhydrous conditions to be maintained and the solvent used has to be completely dried. It is very difficult to maintain such conditions on an industrial scale, and failing to do so leads to low yield of product.
The processes for preparing N-[1-(S)-ethoxy carbonyl-3-phenyl propyl]-L-alanine N- carboxyanhydride which is used in the process of the present invention are well known and are disclosed in JP57175152A, US4496541 , EP215335, US5359086 and EP1197490B1. Trans octahydro-IH-indole-2-carboxylic acid and its esters are the key intermediates in the synthesis of trandolapril. When synthesized, trans octahydro-1 H-indole-2-carboxylic acid is a mixture of four isomers, as shown below.
From the processes known in the prior art, trans octahydro-1 H-indole-2-carboxylic acid is converted to its ester and the ester is then either reacted directly with N-[1-(S)-ethoxy carbonyl-3-phenyl propyl]-L-alanine (ECPPA) and then the isomers are separated by column chromatography, or alternatively the ester is reacted with ECPPA followed by 0 deprotection. Trans octahydro-1 H-indole-2-carboxylic acid is always used in its protected form. No attempts have been made to resolve free trans octahydro-1 H-indole-2-carboxylic acid to convert it to the desired isomer (isomer D, above). Furthermore, none of the prior art processes is stereoselective, so resolution of the required isomer is required following condensation.
EP0088341 and US4490386 describe a method for the resolution of N-benzoyl (2RS,3aR,7aS) octahydro-1 H-indole-2-carboxylic acid using α-phenyl ethyl amine.
US6559318 and EP1140826 describe a process for the synthesis of (2S,3aR,7aS) 0 octahydro-1 H-indole-2-carboxylic acid using enzymatic resolution of its nitrile intermediate. Enzymatic resolution involves many steps and also requires column chromatography for purification making the process uneconomical industrially.
WO8601803 describes the preparation of (2S,3aR,7aS) octahydro-1 H-indole-2-carboxylic 5 acid ethyl ester and benzyl ester using 10-D-camphor sulphonic acid.
WO2004065368 describes the synthesis of (2S,3aR,7aS) octahydro-1 H-indole-2- carboxylic acid benzyl ester by resolution using 10-D-camphor sulphonic acid to prepare trandolapril. This process gives poor yields because the product has to be first resolved and then the ester is deprotected leading to further loss in yield, making the process low yielding and expensive.
W 02005/051909 describes a process for the preparation of trandolapril, i.e. (N-[I-(S)- carbethoxy-3-phenylpropyl}-S-alanyl-2S,3aR,7aS-octahydroindol-2-carboxyIic acid} as well as its pharmaceutical acceptable salts, using a racemic mixture of trans octahydroindole-2- carboxylic acid with the N-carboxyanhydride of {N-[1-(S)-ethoxycarbonyl-3-phenylpropyl}- S-alanyl (NCA) in a molar ratio of 1 :1 to 1.6:1 in a mixture of water and water-miscible solvent to obtain a mixture of diastereomers of trandolapril. The diastereomers are converted to salts which upon repeated crystallization from acetone and water, and reaction with a base gives pure trandolapril. Thus, the condensation reaction in the presence of water and a water-miscible solvent is not stereoselective.
The processes for preparing N-[1-(S)-ethoxy carbonyl-3- phenyl propyl]-l_-alanine N- carboxyanhydride starting from N-[1-(S)-ethoxy carbonyl-3- phenyl propyl]-L-alanine (ECPPA) are well known and are disclosed in JP57175152A, US4496541 , EP215335, US5359086 and EP1197490B1
The angiotensin-converting enzyme (ACE) inhibitor trandolapril is commonly prescribed as a cardiovascular drug for the control and management of mild to severe hypertension Chigh blood pressure) and may be used alone or in combination with diuretics or other antihypertensive agents. Administration of trandolapril is typically oral at a level of around 0.5-4 mg once a 15 day and may also be used in the management of conditions such as heart failure and left ventricular dysfunction following myocardial infarction.
Trandolapril itself is a prodrug, being converted to the acid form “trandolaprilat” in vivo. It is, however, • generally desirable to prepare and administer the ester form.. The structures of trandolapril and trandolaprilat ‘ are shown below.
Trandolapril Trandolaprilat
Various methods for the synthesis of trandolapril and related compounds have been proposed but each of these suffers from drawbacks . Frequently the syntheses require the use of dangerous reagents, which make industrial scale preparation hazardous and difficult and/or involve multiple steps resulting in a long and complex synthesis . One of the most important steps in the synthesis is the formation of the trans-fused octahydroindole ring, which is often difficult to separate from the cis-fused equivalent.
A number of the known synthetic routes to trandolapril proceed via the key intermediate (2S, 3aR,7aS) -octahydro,-lH-indole-2-carboxylic acid. This contains the key trans-fused octahydroindole ring and the correct stereochemistry for the carboxylic acid group at the 2-position. Frequently, these methods require the separation of the cis- and trans-fused rings and, in many cases, resolution of the carboxylate group at the 2 -position is necessary. Where production of the trans-fused ring junction has been possible without generating significant quantities of the cis-product, the syntheses have been long and/or required dangerous reagents such as mercury compounds.
(2S, 3aR, 7aS)-octahydro-lH-indole-2-carboxylic acid
US-A-4691022 gives a synthesis of the above intermediate compound in relatively few steps but requires the trans-octahydroindole as the starting material. The result is also a mixture of the 2-α and 2-β compounds.
EP-A-084164/US-A-4, 933,361 provides an apparently effective method for the synthesis of the cis-fused intermediate beginning with the high-pressure hydrogenation of indole at 100 atmospheres of hydrogen and a platinum catalyst. This document also provides two methods for forming the trans-fused octahydroindole ring, but neither is indicated as being efficient. The first method provides the stereochemistry for the 2 -position from substituted alanine, reacting this with activated cyclohexanone and cyclising the product to give a hexahydroindole . Unfortunately, the reduction of this hexahydroindole to the octahydro- compound produces both cis- and trans-fused product in unknown yield. The second method is to introduce the trans-ring via trans-octahydro-lH-quinolin-2 -one, but no indication of yield in the key step is given and complex series of halogenation, partial re-hydrogenation and re-arrangement are required to reach the desired intermediate .
WO 00/40555 / US 6559318 relies on enzymic resolution of a 2- (2 ‘ , 2 ‘ -methoxyethyl) cyclohexamine with Novozyme7 over 25 hours to provide the N-acetylated (1R, 2S) enantiomer which must then be separated by column chromatography from the. unreacted (IS, 2R) enantiomer. Neither the enzymic resolution nor the chromatography steps are well suited to industrial scale preparations. There are also around ten steps required to reach the desired compound.
The synthetic route to the above octahydroindole intermediate proposed by Henning et al . (Tett. Lett. 24(1983), 5343-5346) quickly and elegantly introduces a 1,2-trans configuration around a cyclohexane ring, but requires the use of mercuric nitrate. The use of mercury compounds is obviously undesirable in the preparation of pharmaceuticals. A further synthesis is provided by Brion et al . (Tett. Lett. 33 (1992) 4889-4892) but it is unclear whether they in fact prepare 5% or 95% of the desired product with 2S stereochemistry. In any case, the method requires eleven steps including an initial pig liver esterase digestion to provide the product in stereochemically pure form but in a 95:5 mixture of isomers at position 2. This method is thus complex and ill suited to industrial scale preparation.
ROUTE A – Separation of enantiomers by the formation of diastereomeric salts with a chiral resolving agent HA* (such as 0, O’ -dibenzoyl-L-tartaric acid), coupling with N- [1- (S) -ethoxycarbonyl-3-phenylpropyl] -L-alanine (ECPPA) derivative and finally deprotecting the carboxylic acid moiety Rλ (such as by hydrogenating a benzyl ester, where Rx = Bn) .
ROUTE B.- Direct reaction of 7A with ECPPA derivative that leads to the formation of diastereoisomers, deprotecting the carboxylic acid moiety and finally separation of diastereoisomers by conventional methods.
1) deprotection >■ trandolapril 2) separation of diastereoisomers
ROUTE C- Treatment of 7A in basic medium and deprotection that leads to the racemic mixture of octahydroindole acid followed by the reaction with ECPPA derivative. This will result in a diastereomeric mixture that can be separated by conventional methods.
COOEtCH,
,1 ) basic medium QC &° ‘ – trandolapril 2) deprotection
2) separation of
racemic diastereoisomers 7A 6C
Route D. Separation of isomers of 6C by conventional methods (i.e. formation of a diastereomeric salt) and coupling with ECCPA derivative.
trandolapril
Route E
This route is an inversion of the steps of route B Firstly the isomers are separated and then the protecting group is removed. 1) separation of diastereoisomers trandolapπl
racemic 2) deprotection
Route F. – The compound 8A is treated to remove the protecting grqup and coupled with an ECPPA derivative,
1) deprotection
2) base treatment
racemic 7A 8A
X activating group
………………………………………………………………………………
http://www.google.com/patents/US20060079698

…………..
INTERMEDIATE
(2S,3aR,7aS)-perhydroindole-2-carboxylic acid (42 g).
IR (Nujol, cm-1): 2923, 2854, 1600, 1458, 1377, 1319. 1H-NMR (D2O): δ 1.1-2.5 (m, 8H), 1.65(m,1H), 1.96-2.37 (m,2H), 2.91(td, 1H),4.46(d, 1H). Mass (m/z): 168.3(M-H).
http://www.faqs.org/patents/app/20110065930
(2S,3aR,7aS)-Octahydro-1H-indole-2-carboxylic acid hydrochloride
yield as a white solid.
1H NMR (D2O, 400 MHz): δ 4.42 (dd, 1H, J=11.1, 2.7 Hz), 2.93, (dt, 1H, J=11.8, 3.6 Hz), 2.36 (ddd, 1H, J=12.9, 6.7, 2.7 Hz), 2.31-2.16 (m, 1H), 2.11-2.01 (m, 2H), 1.92-1.90 (m, 1H), 1.79-1.75 (m, 1H), 1.68-1.53 (m, 2H), 1.34-1.13 (m, 3H);
LC-MS (m/z): 170.1 (M+H).sup.+. The isolated product (5) correlates to the material prepared according to U.S. Pat. No. 487,932 and Tetrahedron Lett., 1992, 33, 4889.
CAS No: 144540-75-0

……………………………………..
REF
Tan, X; He, W; Liu, Y (2009). “Combination therapy with paricalcitol and trandolapril reduces renal fibrosis in obstructive nephropathy”. Kidney international 76 (12): 1248–57. doi:10.1038/ki.2009.346. PMID 19759524.
Urbach, H., Henning, R., Teetz, V., Geiger, R., Becker, R. and Gaul, H. (Hoechst A.G.) Bicyclic amino acid derivatives.DE 3151690, EP 084164, EP 170775.JP 1989301659; JP 1989301695
……………………………………………………………………….
The ESI mass spectrum of the drug trandolapril displayed a molecular ion peak [M+H] + at 431.1 amu. The tandem mass spectra (MS2) showed the fragment ions at m/z 234.2, 170.2, 160.3, 134.2, 130.3, 117.2, 102.3 and 91
The IR spectrum of new impurity showed the following absorption bands 3277cm-1 (NH stretch), 2941cm-1 (aliphatic CH stretch), 1734 and 1653cm-1 (C=O) stretch and 1192cm-1 (C-O stretch)
1H NMR
13 C NMR
TRP = TRANDOLAPRIL COMPARED WITH 2 IMPURITIES
………………………………………………………………………………………
TRANDOLAPRIL SPECTRAL DATA
http://www.google.com.br/patents/US20060079698
IR (KBr, cm-1): 3444, 3280, 2973, 2942, 2881, 1735, 1654, 1456, 1367, 1193, 1024, 699.
The 1H-NMR (CDCl3): δ 7.2 (s, 5H), 4.4(m,4H), 4.2 (q,2H), 3.6-1.3 (m, 18H), 1.28(d+t,6H). CI Mass (m/z): 429.6(M-H).
……………………………………
United States Patent Application 20080171885
http://www.freepatentsonline.com/y2008/0171885.html
M.P.: 122-124° C.,
IR (KBr): 3278.7, 2942.2, 1735.2, 1654.3, 1456.7, 1433.7, 1366.5, 1192.8, 1101.5, 1063.8 and 1023.8 cm−1 (FIG. 1).
1H NMR (CD3OD, δ ppm): 7.33 (s, 5H), 4.34 (m, 3H), 3.86 (q, 2H), 3.28-1.46 (m, 17H) and 1.39 (d+t, 6H),
Mass (m/z, amu): 453.5 (M+Na) and 431.7 (M+H)+ molecular ion.
…………………………………………………………………………….
MORE INFO FOR READERS
trandolapril
Reaction of cyclohexene with acetonitrile and mercuric acetate followed by ligand exchange with sodium chloride gives the crystalline acetamidomercury chloride in 98% yield. Reaction of the product of formula XIIIa with α-chloro acrylonitrile followed by reaction with NaBH4 and ethanol gives the product of formula XIIIb, which is cyclized with sodium in DMF to get a mixture of Xlllc and XIIId in the ratio of 18.5 : 1. On hydrolysis, IIIa is obtained selectively.
In this method, cyclohexylamine derivative of formula-XXIX is resolved to produce enantiomerically pure product of formula XXX, which is converted to octahydroindole-2-carbonitrile of formula XXVIII a. The product of formula XXVIII a on hydrolysis yields the octahydroindole-2-carboxylic of formula III a.

Friday, 6 December 2013
AstraZeneca today announced that the European Commission (EC) has granted Marketing Authorisation to FluenzTM Tetra. Fluenz Tetra is a nasally administered four-strain live attenuated influenza vaccine for the prevention of influenza in children and adolescents from 24 months up to 18 years of age. The EC approval makes Fluenz Tetra the first and only intra-nasal four-strain influenza vaccine available in Europe.http://www.pharmalive.com/ec-approves-fluenz-tetra


WASHINGTON, Dec. 6, 2013 (AP) — Federal health officials have approved a highly anticipated hepatitis C drug from Gilead Sciences Inc. that is expected to offer a faster, more palatable cure to millions of people infected with the liver-destroying virus.
The Food and Drug Administration said Friday it approved the pill Sovaldi in combination with older drugs to treat the main forms of hepatitis C that affect U.S. patients.
Current treatments for hepatitis C can take up to a year of therapy and involve weekly injections of a drug that causes flu-like side effects. That approach only cures about three out of four patients. Sovaldi is a daily pill that in clinical trials cured roughly 90 percent of patients in just 12 weeks, when combined with the older drug cocktail.http://www.pharmalive.com/us-approves-breakthrough-hepatitis-c-drug
Product name: Sovaldi
Common name: Sofosbuvir
Alias: GS-7977, PSI-7977
Chinese name: Sophia Bouvet (Suofabuwei, cable release Wei)
CAS Registry Number :1190307 -88-0
The chemical structure

Indications: Chronic hepatitis C (HCV GT1, GT2, GT3, GT4)
Mechanism: nucleoside NS5B polymerase inhibitor
approved Time: December 6, 2013
, U.S. Patent Number: 7964580,8415322,8334270,7429572;, patent validity: March 26, 2029 (U.S. Patent No.: 7,964,580 and 8,334,270), April 3, 2025 (U.S. Patent No.: 7,429,572 and 8,415,322) Sales value (estimated): $ 1.9 billion (2014), 6600000000 USD (2016) Drug Companies: Gilead Sciences, Inc. (Gilead Sciences)
GS-7977, (S)-isopropyl 2-(((S)-(((2R,3R,4R,5R)-5-(2,4-dioxo-3,4- dihydropyrimidin^l(2H)-yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran-2- yl)methoxy)(phenoxy)phosphoryl)amino)propanoate, available from Gilead Sciences, Inc., is described and claimed in U.S. Patent No. 7,964,580. (See also US 2010/0016251, US 2010/0298257, US 201 1/0251 152 and US 2012/0107278.) GS-7977 has the structure:
GS-7977 can be crystalline or amorphous. Examples of preparing crystalline and amorphous forms of GS-7977 are disclosed in US 2010/0298257 (US 12/783,680) and US 201 1/0251 152 (US 13/076,552), both of which are incorporated by reference.

…………

Tandospirone, [112457-95-1]
US 5011841
(lR*,2S*,3R*,4S*)-N-[4-[4-(2- US 5011841 citrate Pyrimidinyl) piperazin-1-
yl] butyl ] -2 , 3-norbornane- dicarboximide citrate
Tandospirone hyd, SM-3997,
Chemical Name: (3aR,4S,7R,7aS)-rel-Hexahydro-2-[4-[4-(2-pyrimidinyl)-1-piperazinyl]butyl]-4,7-methano-1H-isoindole-1,3(2H)-dione hydrochloride
Tandospirone (Sediel), also known as metanopirone, is an anxiolytic andantidepressant used in China and Japan, where it is marketed by Dainippon Sumitomo Pharma. It is a member of the azapirone and piperazine chemical classes and is closely related to other agents like buspirone and gepirone.
Tandospirone is most commonly used as a treatment for anxiety and depressive disorders, such as generalised anxiety disorder and dysthymia respectively.[1] For both indications it usually takes a couple of weeks for therapeutic effects to be start being seen,[1] although at higher doses more rapid anxiolytic responses have been seen.[2] It has also been used successfully as a treatment for bruxism.[3]
Tandospirone has also been tried, successfully, as an adjunctive treatment for cognitive symptoms in schizophrenic individuals.[4]
It is not believed to be addictive but it is known to produce mild withdrawal effects (e.g. anorexia) after abrupt discontinuation.[1]
Yevich, Joseph P.; New, James S.; Smith, David W.; Lobeck, Walter G.; Catt, John D.; Minielli, Joseph L.; Eison, Michael S.; Taylor, Duncan P. et al. (1986). “Synthesis and biological evaluation of 1-(1,2-benzisothiazol-3-yl)- and (1,2-benzisoxazol-3-yl)piperazine derivatives as potential antipsychotic agents”. Journal of Medicinal Chemistry 29 (3): 359–69. doi:10.1021/jm00153a010.PMID 2869146.
Tandospirone acts as a potent and selective 5-HT1A receptor partial agonist, with a Ki affinity value of 27 ± 5 nM[5] and approximately 55-85% intrinsic activity.[6][7] It has weak and clinically negligible affinity for the 5-HT2A (1,300 ± 200), 5-HT2C (2,600 ± 60), α1-adrenergic (1,600 ± 80), α2-adrenergic (1,900 ± 400), D1 (41,000 ± 10,000), and D2 (1,700 ± 300) receptors, and is essentially inactive at the 5-HT1B, 5-HT1D, β-adrenergic, and muscarinic acetylcholine receptors, serotonin transporter (SERT), and benzodiazepine (BDZ)allosteric site of the GABAA receptor (all of which are > 100,000).[5] There is evidence of tandospirone having low but significantantagonistic activity at the α2-adrenergic receptor through its active metabolite 1-(2-pyrimidinyl)piperazine (1-PP), however.[8][9]
close analogs. The halide, X, is preferably bromide. The reaction is carried out in a hot inert reaction medium in the presence of an acid scavenging base. In practice, the reaction process involves a multiphasic reaction of (3) and (4) in refluxing xylene with an excess of solid potassium carbonate.
such as imidate anions of structure (2a),
wherein MIB represents an alkali or alkaline earth metal, can be reacted with a pyrimidinylpiperazinyl derivative of formula (5),
wherein Q is a nucleofuge, i.e. a leaving group of the type commonly utilized in synthetic organic chemistry; by heating in an inert solvent under standard conditions such as those described for the alkylation step of the Gabriel synthesis; cf: Gibson and Bradshaw, Angew. Chem. Int. Ed., 7/919,930, (1968).
Tandospirone and tandospirone salts have been described in several patents and patent applications. These describe pharmaceutical compositions of tandospironealone and in combination with other drugs for treatment of human disease and include EP 0437026 (Treatment of depression), WO 1994016699 (Compositions containing tandospirone or its analogues), EP 0082402 (Succinimide derivates and process for preparation thereof), JP 2002020291 (Therapeutic agents for cognition disorders), JP 2003335678 (Therapeutic agents for neurogenic pain), WO 2004002487 (Methods for treating attention deficit disorder), JP 2005225844 (Agents for the treatment of irritable bowel syndrome), WO 20051 17886 (Adhesive patch),WO 2008044336 (Crystal- containing adhesive preparation) and WO 2010065730 (Pharmaceutical suspension).
………………………………………
Drugs Fut 1986,11(11),949
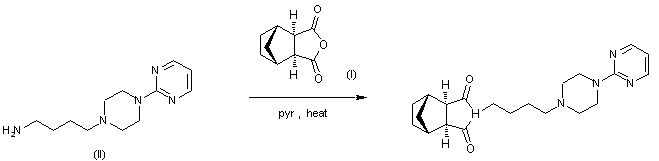
By condensation of norbornane-2,3-di-endo-carboxylic anhydride (I) with 1-(4-aminobutyl)-4-(2 pyrimidinyl)piperazine (II) in refluxing pyridine.
……………………………………………………….
CA 1184915; EP 0082402
The cyclization of methyl 3-[4-(4-methoxybenzoylamino)-3-nitrobenzoyl]butyrate (I) with hydrazine hydrate in refluxing acetic acid gives 4,5-dihydro-6-[4-(4-methoxybenzoylamino)-3-nitrobenzoyl]-5-methylpyridazin-3(2H)-one (II), which is reduced with H2 over Pd/C in ethanol yielding the corresponding amino derivative (III). Finally, this compound is cyclized in refluxing acetic acid.
……………………………………………………..
Chem Pharm Bull 1991,39(9),2288
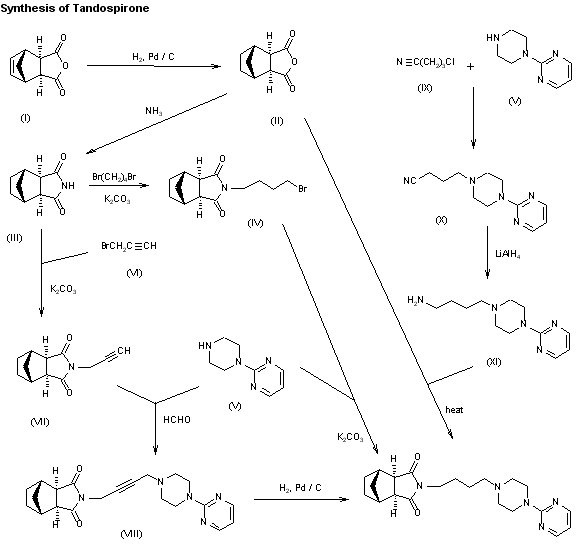
New syntheses of tandospirone have been described: 1) The hydrogenation of bicyclo[2.2.1]hept-5-ene-2,3-di-exo-carboxylic acid anhydride (I) with H2 over Pd/C in THF-water gives bicyclo[2.2.1]heptane-2,3-di-exo-carboxylic acid anhydride (II), which by reaction with ammonia in THF-water is converted into the imide (III). The reaction of (III) with 1,4-dibromobutane by means of K2CO3 in refluxing acetone yields N-(4-bromobutyl)bicyclo[2.2.1]heptane-2,3-di-exo-carboximide (IV), which is finally condensed with 1-(2-pyrimidinyl)piperazine (V) by means of K2CO3 and KI in hot DMF. 2) Imide (III) is condensed with propargyl bromide (VI) by means of K2CO3 in refluxing acetone affording N-propargylbicyclo[2.2.1]heptane-2,3-di-exo-carboximide (VII), which is allowed to react with piperazine (V) and formaldehyde by means of CuSO4 in dioxane to give N-[4-[4-(2-pyrimidinyl)piperazin-1-yl]-2-butynyl]bicyclo[2.2.1]heptane-2,3-di-exo-carboximide (VIII). Finally, this compound is reduced with H2 over Pd/C. 3) The condensation of piperazine (V) with 4-chlorobutyronitrile (IX) by means of NaOH in acetone gives 4-[4-(2-pyrimidinyl)piperazin-1-yl]butyronitrile (X), which is reduced with LiAlH4 in ether yielding 1-(4-aminobutyl)-4-(2-pyrimidinyl)piperazine (XI). Finally, this compound is condensed with anhydride (II) in refluxing pyridine.
………………….



…………

PITAVASTATIN, LIVALO, Itavastatin calcium, Nisvastatin, NKS-104, NK-104,
CAS REGISTRY NUMBER
rotation is +
alpha(D20) +6.8° (c 1.74, CHCl3)
ALSO
Bioorganic and Medicinal Chemistry Letters, 1999 , VOL 9, 20 pg. 2977 – 2982…….alpha(D20) +23.1° (c 1.0, acn/water(1:))
Helvetica Chimica Acta, 2007 , vol. 90, 6 pg. 1069 – 1081…alpha(D20) +22.9° (c 1.0, acn/water)
(3R,5S,6E)-7-[2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl]-3,5-dihydroxyhept-6-enoic acid
Pitavastatin a lipid-lowering agent that belongs to the statin class of medications for treatment of dyslipidemia. It is also used for primary and secondary prevention of cardiovascular disease. FDA approved in Aug 3, 2009.
2-C25-H23-FN-O4.Ca, 881.01
Statin drugs are currently the most therapeutically effective drugs available for reducing the level of Low density lipoprotein (LDL) in the blood stream of a patient at risk for cardiovascular disease. A high level of LDL in the
bloodstream has been linked to the formationof coronary lesions which obstruct the flow of blood and can rupture and promote thrombosis. It is well known that inhibitors against HMG CoA reductase which is rate limiting enzyme for cholesterol biosynthesis have been clinically proved to be potentially useful anti-hyperlipoproteinemic agents
and they are considered very effective curative and preventive for coronary artery sclerosis or atherosclerosis .
Pitavastatin calcium was discovered by Nissan Chemical Industries Limited Japan and developedfurther by Kowa Pharmaceuticals Tokyo Japan is a novel member of the medication class of statins.
LIVALO (pitavastatin) is an inhibitor of HMG-CoA reductase. It is a synthetic lipid-lowering agent for oral administration.
The chemical name for pitavastatin is (+)monocalcium bis{(3R, 5S, 6E)-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl]-3,5dihydroxy-6-heptenoate}. The structural formula is:
 |
The empirical formula for pitavastatin is C50H46CaF2N2O8 and the molecular weight is 880.98. Pitavastatin is odorless and occurs as white to pale-yellow powder. It is freely soluble in pyridine, chloroform, dilute hydrochloric acid, and tetrahydrofuran, soluble in ethylene glycol, sparingly soluble in octanol, slightly soluble in methanol, very slightly soluble in water or ethanol, and practically insoluble in acetonitrile or diethyl ether. Pitavastatin is hygroscopic and slightly unstable in light.
Each film-coated tablet of LIVALO contains 1.045 mg, 2.09 mg, or 4.18 mg of pitavastatin calcium, which is equivalent to 1 mg, 2 mg, or 4 mg, respectively of free base and the following inactive ingredients: lactose monohydrate, low substituted hydroxypropylcellulose, hypromellose, magnesium aluminometasilicate, magnesium stearate, and film coating containing the following inactive ingredients: hypromellose, titanium dioxide, triethyl citrate, and colloidal anhydrous silica.
Pitavastatin (usually as a calcium salt) is a member of the blood cholesterol loweringmedication class of statins,[1] marketed in the United States under the trade nameLivalo. Like other statins, it is an inhibitor of HMG-CoA reductase, the enzyme that catalyses the first step of cholesterol synthesis. It has been available in Japan since 2003, and is being marketed under licence in South Korea and in India.[2] It is likely that pitavastatin will be approved for use in hypercholesterolaemia (elevated levels of cholesterol in the blood) and for the prevention of cardiovascular disease outside South and Southeast Asia as well.[3] In the US, it received FDA approval in 2009.[4]
Pitavastatin is used to lower serum levels of total cholesterol, LDL-C, apolipoprotein B, and triglycerides, and raise levels of HDL-C for the treatment of dyslipidemia.
Like the other statins, pitavastatin is indicated for hypercholesterolaemia (elevated cholesterol) and for the prevention of cardiovascular disease. A 2009 study showed that pitavastatin increased HDL cholesterol (24.6%), especially in patients with HDL lower than 40 mg/dl, in addition to greatly reducing LDL cholesterol (–31.3%).[5] As a consequence, pitavastatin is most likely to be appropriate for patients with metabolic syndrome with high LDL, low HDL and diabetes mellitus.
Common statin-related side effects (headaches, stomach upset, abnormal liver function tests and muscle cramps) were similar to other statins. However, pitavastatin seems to lead to fewer muscle side effects than certain statins that are lipid-soluble, as a result of the fact that pitavastatin is water-soluble (as is pravastatin, for example).[6] One study found that coenzyme Q10 was not reduced as much as with certain other statins (though this is unlikely given the inherent chemistry of the HMG-CoA reductase pathway that all statin drugs inhibit).[3][7]
Hyperuricemia or increased levels of serum uric acid have been reported with pitavastatin.[8]
Most statins are metabolised in part by one or more hepatic cytochrome P450enzymes, leading to an increased potential for drug interactions and problems with certain foods (such as grapefruit juice). Pitavastatin appears to be a substrate ofCYP2C9, and not CYP3A4 (which is a common source of interactions in other statins). As a result, pitavastatin is less likely to interact with drugs that are metabolized via CYP3A4, which might be important for elderly patients who need to take multiple medicines.[3]
Pitavastatin (previously known as itavastatin, itabavastin, nisvastatin, NK-104 or NKS-104) was discovered in Japan by Nissan Chemical Industries and developed further byKowa Pharmaceuticals, Tokyo.[3] Pitavastatin was approved for use in the United States by the FDA on 08/03/2009 under the trade name Livalo. Pitavastatin has been also approved by the Medicines and Healthcare products Regulatory Agency (MHRA) in UK on 17 August 2010.
![]()

|
Country
|
Patent Number
|
Approved
|
Expires (estimated)
|
|---|---|---|---|
| United States | 7,022,713 | 2009-08-03 | 2024-02-19 |
| United States | 6,465,477 | 2009-08-03 | 2016-12-20 |
| United States | 5,856,336 | 2009-08-03 | 2016-01-05 |
| United States | 5,854,259 | 2009-08-03 | 2015-12-29 |
| United States | 5,753,675 | 2009-08-03 | 2015-05-19 |
JP 1993310700, JP 1994025092
Tetrahedron Lett 1993, 34, 51, 8263-6.
Bioorg Med Chem2001, 9, (10): 2727
Drugs Fut1998, 23, (8) :847-859
Bull Chem Soc Jpn1995, 68, (1) :364-72
Tetrahedron Asymmetry1993, 4, (2) :201-4
Tetrahedron Lett1993, 34, (51) :8267-70
A Endo; J. Med. Chem. 1985, 28, 401.
AM Gotta; LC Smith; IXth International Symp. Drugs Affecting Lipid Metabolism, 1986, 30- 31
Y Fujikawa, Nissan Chemical Industries Ltd, EP304063 (A3), 1989.
S Ahmed; CS Madsen; PD Stein; J. Med. Chem. 2008, 51, 2722-2733.
K Turner; Org. Process. Res. Dev, 2004, 8, 823-833.
Z Casar; M Steinbocher; J Kosmrlj; J. Org. Chem. 2010, 75(19), 6681-6684.
KL Baumann; Tetrahedron Letters, 1992, 33, 2283-2284.
N Miyachi; Y Yanagawa; H Iwasaki; Tetrahedron Lett. 1993, 34, 8267-8270.
T Minami; K Takahashi; T Hiyama; Tetrahedron Lett. 1993, 34, 3, 513-516.
DA Evans; AH Hoveyda; J. Org. Chem. 1990, 55, 5190-5192.
J Castorer; LA Sorbera; PA Leeson; Drugs Fut. 23(8), 1998, 847-859.
T Hiyama; K Takahashi; T Minami; Bull. Chem. Soc. Jpn. 1995, 68, 364-372.
MS Reddy; M Bairy; K Reddy; Oriental Journal of Chemistry. 2007, 23, 559-564.
RN Moore; G Bigam; JK Chan; AM Hogg; JC Vederas; J. Am. Chem. Soc. 1985, 107 3694-3701.
DS Johnson; JJ Li; Art of Drug Synthesis, John Wiley & Sons, New Jersey, 2007, 177-181.
MT Stone; Organic Lett. 2011, 13, 2326-2329.
SR Manne, SR Maramreddy, WO2007132482 (A2), 2007.
SD Dwivedi, DJ Patel, AP Shah, Cadila Healthcare Ltd, US0022102 (A1), 2012.
……………………………………………………..

……………………………

The reaction of 1 (R) ,7,7-trimethylbicyclo [2.2.1] heptan-2-one (I) with 1 -naphthylmagnesium bromide (II) gives the tertiary alcohol (III), which by reaction with SOCl2 and then with NaHCO3 yields 2 – (1-naphthyl) -1 (R) ,7,7-trimethylbicyclo [2.2.1] heptene (IV ). Hydroboration of (IV) with BH3 followed by oxidation with H2O2 affords 4 (S) ,7,7-trimethyl-3exo-(1-naphthyl) bicyclo [2.2.1] heptan-2exo-ol (V), which is submitted to transesterification with methyl acetoacetate (VI) and dimethyl-aminopyridine (DMAP) to give the corresponding ester (VII). The condensation of (VII) with N-methoxy-N-methyl-3-[2-cyclopropyl-4-( 4-fluorophenyl) quinolin-3-yl] -2 (E)-propenamide (VIII) by means of NaH yields the corresponding chiral 3,5-dioxoheptenoic acid ester (IX), which is selectively reduced first with diisobutylaluminum hy-dride acid (DIBAL) and then with diethylmethoxyborane and sodium borohydride affording the 3 (R), 5 (S)-dihydroxyheptenoic ester (X). Finally, this compound is saponified with NaOH and treated with acetic acid / sodium acetate. The intermediate amide (VIII ) is obtained by condensation of 2-cyclopropyl-4-(4-fluorophenyl) quinoline-3-carbaldehyde (XI) with N-methoxy-N-methylacetamide (XII) by means of butyllithium to the hydroxy propionamide (XIII), which is then dehydrated with methanesulfonyl chloride and triethylamine in the usual way).
…………………
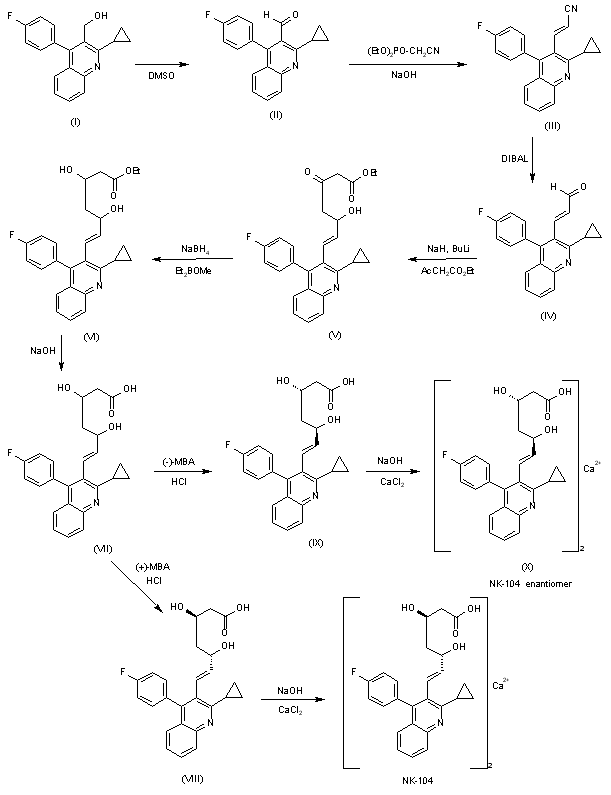
A systematic chiral synthesis of NK-104 and its enantiomer (X) has been reported: The oxidation of the already known 2-cyclopropyl-4-(4-fluorophenyl)quinoline-3-methanol (I) with DMSO, P2O5 and triethylamine gives the corresponding aldehyde (II), which is condensed with diethyl cyanomethylphosphonate by means of NaOH in toluene yielding the propenenitrile (III). The reduction of (III) with DIBAL affords the unsaturated aldehyde (IV), which is condensed with ethyl acetoacetate by means of NaH and n-BuLi to provide the 3-oxo-5-hydroxy-6-heptenoic acid ethyl ester derivative (V). The highly syn stereoselective reduction of (V) by means of diethylmethoxyborane and NaBH4 yields the desired syn racemic mixture of erythro-beta,delta-dihydroxyesters (VII), which is submitted to optical resolution with chiral (+)-alpha-methylbenzylamine [(+)-MBA] to obtain NK-104 free acid (VIII), which is finally treated with NaOH and CaCl2. The enantiomer of NK-104 has been obtained by optical resolution of the racemic mixture (VII) with (-)-alpha-methylbenzylamine to obtain the enantiomeric free acid (IX), which is treated with NaOH and CaCl2 as before.
Fujikawa, Y.; Suzuki, M.; Iwasaki, H.; Kitahara, M.; Sakashita, M.; Sakoda, R.;. Synthesis and biological evaluations of quinolone-based HMG-CoA reductase inhibitors Bioorg Med Chem 2001, 9 , 10, 2727
………
![]()
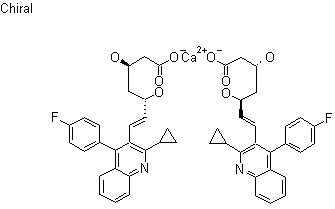
ADDITIONAL UPDATED INFO
Pitavastatin calcium is a novel member of the medication class of statins. Marketed in the United States under the trade name Livalo, it is like other statin drugs an inhibitor of HMG-CoA reductase, the enzyme that catalyses the first step of cholesterol synthesis. It is likely that pitavastatin will be approved for use in hypercholesterolaemia (elevated levels of cholesterol in the blood) and for the prevention of cardiovascular disease outside South and Southeast Asia as well.
Pitavastatin calcium is chemically known as (3R,5S)-7-[2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl]-3,5-dihydroxy-6(E)-heptenoic acid calcium salt having the formula IA is known in the literature.
Pitavastatin is a synthetic lipid-lowering agent that acts as an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme a (HMG-CoA) reductase (HMG-CoA Reductase inhibitor). This enzyme catalyzes the conversions of HMG-CoA to mevalonate, inhibitors are commonly referred to as “statins”. Statins are therapeutically effective drugs used for reducing low density lipoprotein (LDL) particle concentration in the blood stream of patients at risk for cardiovascular disease. Pitavastatin is used in the treatment of hyperchloesterolemia and mixed dyslipidemia.
Pitavastatin calcium has recently been developed as a new chemically synthesized and powerful statin by Kowa Company Ltd, Japan. On the basis of reported data, the potency of Pitavastatin is dose-dependent and appears to be equivalent to that of Atorvastatin. This new statin is safe and well tolerated in the treatment of patients with hypercholesterolaemia. Significant interactions with a number of other commonly used drugs can be considered to be extremely low.
Pitavastatin was disclosed for the first time in US patents US 4,761,419, US 5,01 1 ,930 and US 5,753,675. The process disclosed in these patents for the preparation of Pitavastatin is as shown below:
wherein R is hydrogen or protecting group.
US 5,284,953 discloses a process for the preparation of Pitavastatin calcium, which employs optically active a-methylbenzylamine as a resoluting agent.
The above processes are economically not viable, as resolution is carried out in final stage.
US 6,835,838 B2 discloses a process for the preparation of Pitavastatin calcium, which is as shown below:
However, it has been observed that the above process of lactonization results in ~10- 15% of unreacted Pitavastatin ethyl ester and therefore results in low yield. Further, -10% of Pitavastatin acid results during the above lactonization process and therefore does not produce a single product which is required to keep adequate control for an intermediate through specifications to have consistently better quality of the finished product.
Processes for the preparation of Pitavastatin are described in EP-A-0304063 and EP-A-1099694 and in the publications by N. Miyachi et al. in Tetrahedron Letters (1993) vol. 34, pages 8267-8270 and by K. Takahashi et al. in Bull. Chem. Soc. Japan (1995) Vol. 68, 2649-2656. These publications describe the synthesis of Pitavastatin in great detail but do not describe the hemi-calcium salt of Pitavastatin. The publications by L A. Sorbera et al. in Drugs of the Future (1998) vol. 23, pages 847-859 and by M. Suzuki at al. in Bioorganic & Medicinal Chemistry Letters (1999) vol. 9, pages 2977-2982 describe Pitavastatin calcium, however, a precise procedure for its preparation is not given. A full synthetic procedure for the preparation of Pitavastatin calcium is described in EP-A-0520406. In the process described in this patent Pitavastatin calcium is obtained by precipitation from an aqueous solution as a white crystalline material with a melting point of 190-192° C.
US20090182008 A1 discloses polymorphic form A, B, C, D, E, and F, and the amorphous form of Pitavastatin Calcium salt (2:1). In particular, crystalline Form A having water content from about. 5% to about 15% and process for its preparation are disclosed.
US20090176987 A1 also discloses polymorphic form crystal form A of Pitavastatin Calcium which contains from 5 to 15% of water and which shows, in its X-ray powder diffraction as measured by using CuKa radiation, a peak having a relative intensity of more than 25% at a diffraction angle (20) of 30.16°.
WO2007/132482 A1 discloses a novel process for the preparation of Pitavastatin Calcium by condensing bromide salt of formula-3 with aldehyde compound of formula-4 to obtain olefinic compound of formula-5 and converting olefinic compound to Pitavastatin Calcium via organic amine salt for purification.
Pitavastatin and its process were disclosed in U.S. Pat. No. 5,753,675.
Pitavastatin calcium and its process were disclosed in U.S. Pat. No. 5,856,336. PCT publication no. WO 2004/072040 (herein after referred to ’040 patent) disclosed crystalline polymorph A, polymorph B, polymorph C, polymorph D, polymorph E, polymorph F and amorphous form of pitavastatin calcium
WO 1995/1 1898 Al discloses a process for the preparation of Pitavastatin, which is as shown below:
wherein Y represents P+RnRi2Ri3Hal“ or P(W)Ri4R15; R9a, R% and R]0 are protecting groups each of Rn, Rj2> R^, Ri4 and R15 which are independent of one another, is optionally substituted alkyl or optionally substituted aryl group; R14 and Rj5 together form a 5- or 6-membered ring; Hal is chlorine, bromine or iodine; and W is O or S.
The above process results in 2-5% of Cis isomer of Pitavastatin which requires further purification and therefore results poor yield.
US 6,875,867 B2 discloses a process for the preparation of Pitavastatin arginine salt, which is as shown below:
Saponification / Base
During the above process Trifluoroacetic acid or hydrochloric acid is used to break the acetonide and the Pitavastatin ester formed is converted in situ to its corresponding alkali salt by treating with base, such as sodium hydroxide.
US20090182008 A1 discloses polymorphic form A, B, C, D, E, and F, and the amorphous form of Pitavastatin Calcium salt (2:1). In particular, crystalline Form A having water content from about. 5% to about 15% and process for its preparation are disclosed.
……………………………………..
nmr
http://scholarsresearchlibrary.com/dpl-vol4-iss5/DPL-2012-4-5-1553-1557.pdf
calcium bis-(E)-3,5-dihydroxy-7-[4’-(4’’-flurophenyl)-2’-
cyclopropyl-quinoline-3-yl]-hept-6-enoate , pitavastatin calcium
Melting Point: 207 degC;
IR υmax (KBr) cm-1: 3366 (OH), 2911, 1603 (C=O), 1567 (C=N), 1513 (C=C),
1488 (C-H), 1416 (C-H), 1313, 1275, 1221 (C-O-C), 1158, 1065 (C-H), 972, 843, 763.
1H-NMR (500MHz, DMSO-d6):
δ 1.01 (m, 2H), 1.09 (m, 1H), 1.19 (m, 2H), 1.41 (m, 1H),
1.98 (dd, 1H, J1 =8.5,
J2 =15.5Hz), 2.11(d, 1H, J1 =3.0, J2 =15.5Hz), 2.50 (m, 2H),
3.66 (m, 1H), 4.13 (m, 1H), 4.95 (s, 1H), 5.58 (dd, 1H,
J1 =5.5, J2 =10.5Hz), 6.49 (d, 1H, J = 16.0Hz),
7.35 (m, 6H), 7.59 (m, 1H, J = 7.0Hz), 7.83 (d, 1H, J =8.5Hz).
13CNMR & DEPT (125.76MHz, DMSO-d6):
δ 11.12(CH2, C-17), 11.23(CH2,C-18), 15.80(CH2, C-16), 44.29(CH2,
C-22), 44.61(CH2, C-24), 66.61(C-O, C-23),
69.34(C-O,C-21),115.53(C=C, C-20), 15.62(CH), 115.79(CH),
123.59(CH), 126.07(C=C, C-19),
128.79(CH),129.20(CH),130.07(CH), 32.30(CH),
132.56(CH), 133.51(C),
142.60(C), 144.09(C), 146.37(C),
161.02(C), 163.00(C), 179.13(C=O, C-25).
ESI-MS: m/z (%) 318 (100), 274 (23), 423 (13), 422 (M+, 70); EI calcd for C25H24FNO4, 421.461; found, 422.220
(M+).
…………………
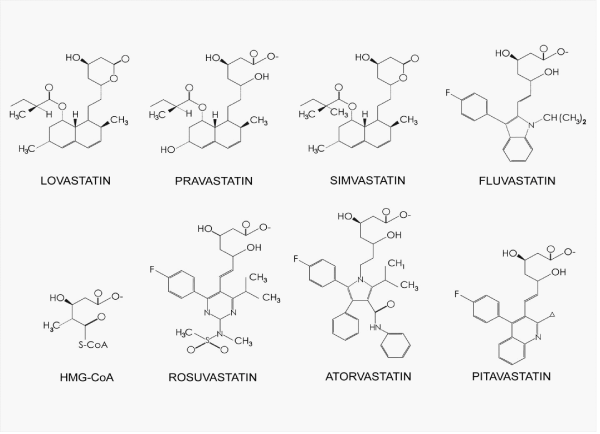
………………![]()


(2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethylethyl)-4-hydroxy-7-{[4-methoxy-3-(3-methoxypropoxy)phenyl]methyl}-8-methyl-2-(propan-2-yl)nonanamide, 173334-57-1, base
173334-58-2,aliskiren hemifumarate
Aliskiren is a renin inhibitor. It was approved by the U.S. Food and Drug Administration in 2007 for the treatment of hypertension.
Tekturna contains aliskiren hemifumarate, a renin inhibitor, that is provided as tablets for oral administration. Aliskiren hemifumarate is chemically described as (2S,4S,5S,7S)-N-(2-carbamoyl-2-methylpropyl)-5-amino-4-hydroxy-2,7diisopropyl-8-[4-methoxy-3-(3-methoxypropoxy)phenyl]-octanamide hemifumarate and its structural formula is
 |
Molecular formula: C30H53N3O6 • 0.5 C4H4O4
Aliskiren hemifumarate is a white to slightly yellowish crystalline powder with a molecular weight of 609.8 (free base- 551.8). It is soluble in phosphate buffer, n-octanol, and highly soluble in water.
……………………………….
|
……….
Aliskiren (INN) (trade names Tekturna, US; Rasilez, UK and elsewhere) is the first in a class of drugs called direct renin inhibitors. Its current licensed indication is essential (primary) hypertension.
Aliskiren was co-developed by the Swiss pharmaceutical companies Novartis andSpeedel.[1][2] It was approved by the US Food and Drug Administration in 2007 for the treatment of primary hypertension.[3]
In December 2011, Novartis had to halt a clinical trial of the drug after discovering increased incidence of nonfatal stroke, renal complications, hyperkalemia, and hypotension in patients with diabetes and renal impairment (ALTITUDE Trial ).[4] [5]
As a result, in April 20, 2012:
Renin, the first enzyme in the renin-angiotensin-aldosterone system, plays a role in blood pressure control. It cleaves angiotensinogen to angiotensin I, which is in turn converted byangiotensin-converting enzyme (ACE) to angiotensin II. Angiotensin II has both direct and indirect effects on blood pressure. It directly causes arterial smooth muscle to contract, leading to vasoconstriction and increased blood pressure. Angiotensin II also stimulates the production of aldosterone from the adrenal cortex, which causes the tubules of the kidneys to increase reabsorption of sodium, with water following, thereby increasing plasma volume, and thus blood pressure. Aliskiren binds to the S3bp binding site of renin, essential for its activity.[6] Binding to this pocket prevents the conversion of angiotensinogen to angiotensin I. Aliskiren is also available as combination therapy withhydrochlorothiazide.[7]
Many drugs control blood pressure by interfering with angiotensin or aldosterone. However, when these drugs are used chronically, the body increases renin production, which drives blood pressure up again. Therefore, doctors have been looking for a drug to inhibit renin directly. Aliskiren is the first drug to do so.[8][9]
Aliskiren may have renoprotective effects independent of its blood pressure−lowering effect in patients with hypertension, type 2 diabetes, and nephropathy, who are receiving the recommended renoprotective treatment. According to the AVOID study, researchers found that treatment with 300 mg of aliskiren daily, as compared with placebo, reduced the mean urinary albumin-to-creatinine ratio by 20%, with a reduction of 50% or more in 24.7% of the patients who received aliskiren as compared with 12.5% of those who received placebo. Furthermore, the AVOID trial showed treatment with 300 mg of aliskiren daily reduces albuminuria in patients with hypertension, type 2 diabetes, and proteinuria, who are receiving the recommended maximal renoprotective treatment with losartan and optimal antihypertensive therapy. Therefore, direct renin inhibition will have a critical role in strategic renoprotective pharmacotherapy, in conjunction with dual blockade of the renin−angiotensin−aldosterone system with the use of ACE inhibitors and angiotensin II–receptor blockers, very high doses of angiotensin II−receptor blockers, and aldosterone blockade.[10]
Aliskiren is a minor substrate of CYP3A4 and, more important, P-glycoprotein:
Drugs Fut2001, 26, (12): 1139
Tetrahedron Lett 2001, 42: 4819-23.
Tetrahedron Lett2000, 41, (51): 10085
EP 0678500; EP 0678503; JP 1996053434; JP 1996081430; US 5559111; US 5627182; US 5646143, WO 0109079; WO 0109083
…………………….
………………………….

…………………………………….
NMR
ALISKIREN BASE

MS m/z: 552.6 (M+H)+; 1H-NMR (400 MHz, CDCl3) δ 6.88-6.75 (m, 3H), 4.08-4.04 (t, J = 6.3Hz, 2H), 3.79 (s, 3H), 3.60-3.55 (t, J = 6.3Hz, 2H), 3.30 (s, 3H), 3.30-3.25 (m, 3H), 2.69 (m, 2H), 2.49 (m, 1H), 2.27 (m, 1H), 2.04 (m, 2H), 1.78-1.35 (m, 7H), 1.10 (m, 6H), 0.90 (m, 12H) ppm.

TOFOGLIFLOZIN
CSG-452
R-7201
RG-7201
CAS..1201913-82-7
(1S,3′R,4′S,5′S,6′R)-6-(4-Ethylbenzyl)-6′-(hydroxymethyl)-3′,4′,5′,6′-tetrahydro-3H-spiro[2-benzofuran-1,2'-pyran]-3′,4′,5′-triol hydrate (1:1)
THERAPEUTIC CLAIM Treatment of diabetes mellitus
CHEMICAL NAMES
1. Spiro[isobenzofuran-1(3H),2'-[2H]pyran]-3′,4′,5′-triol, 6-[(4-ethylphenyl)methyl]-3′,4′,5′,6′-tetrahydro-6′-(hydroxymethyl)-, hydrate (1:1), (1S,3′R,4′S,5′S,6′R)-
2. (1S,3′R,4′S,5′S,6′R)-6-[(4-ethylphenyl)methyl]-6′-(hydroxymethyl)-3′,4′,5′,6′-tetrahydro-3H-spiro[2-benzofuran-1,2'-pyran]-3′,4′,5′-triol monohydrate
3. (1S,3′R,4′S,5′S,6′R)-6-[(4-ethylphenyl)methyl]-3′,4′,5′,6′-tetrahydro-6′-(hydroxymethyl)-
spiro[isobenzofuran-1(3H),2'-[2H]pyran]-3′,4′,5′-triol monohydrate
MOLECULAR WEIGHT 404.5
SPONSOR Chugai Pharmaceuticals
CODE DESIGNATION CSG452
Tofogliflozin (USAN, codenamed CSG452) is an experimental drug for the treatment ofdiabetes mellitus and is being developed by Chugai Pharma in collaboration with Kowa andSanofi.[1] It is an inhibitor of subtype 2 sodium-glucose transport protein (SGLT2), which is responsible for at least 90% of the glucose reabsorption in the kidney. As of September 2012, the drug is in Phase III clinical trials.[2][3]
Tofogliflozin is the name of the monohydrate, which is the form used as a drug. The active moiety or anhydrous form (ChemSpider ID: 28530778, CHEMBL2110731) has the chemical formula C22H26O6 and a molecular mass of 386.44 g/mol.[4]
SGLT2 inhibitors inhibitors represent a novel class of agents that are being developed for the treatment or improvement in glycemic control in patients with type 2 diabetes. Glucopyranosyl-substituted benzene derivative are described in the prior art as SGLT2 inhibitors, for example in
WO 01/27128, WO 03/099836, WO 2005/092877, WO 2006/034489,
WO 2006/064033, WO 2006/117359, WO 2006/117360,
WO 2007/025943, WO 2007/028814, WO 2007/031548,
WO 2007/093610, WO 2007/128749, WO 2008/049923, WO 2008/055870, WO 2008/055940.
The glucopyranosyl-substituted benzene derivatives are proposed as inducers of urinary sugar excretion and as medicaments in the treatment of diabetes.
The term “canagliflozin” as employed herein refers to canagliflozin, including hydrates and solvates thereof, and crystalline forms thereof and has the following structure:
The compound and methods of its synthesis are described in WO 2005/012326 and WO 2009/035969 for example. Preferred hydrates, solvates and crystalline forms are described in the patent applications WO 2008/069327 for example.
atigliflozin, including hydrates and solvates thereof, and crystalline forms thereof and has the following structure:
The compound and methods of its synthesis are described in WO 2004/007517 for example.
ipragliflozin, including hydrates and solvates thereof, and crystalline forms thereof and has the following structure:
The compound and methods of its synthesis are described in WO 2004/080990, WO 2005/012326 and WO 2007/114475 for example.
tofogliflozin, including hydrates and solvates thereof, and crystalline forms thereof and has the following structure:
The compound and methods of its synthesis are described in WO 2007/140191 and WO 2008/013280 for example.
remogliflozin and prodrugs of remogliflozin, in particular remogliflozin etabonate, including hydrates and solvates thereof, and crystalline forms thereof. Methods of its synthesis are described in the patent applications EP 1213296 and EP 1354888 for example.
sergliflozin and prodrugs of sergliflozin, in particular sergliflozin etabonate, including hydrates and solvates thereof, and crystalline forms thereof. Methods for its manufacture are described in the patent applications EP 1344780 and EP 1489089 for example.
luseoghflozin, including hydrates and solvates thereof, and crystalline forms thereof and has the following structure:
ertugliflozin, including hydrates and solvates thereof, and crystalline forms thereof and has the following structure:
and is described for example in WO 2010/023594.
The compound of the formula
is described for example in WO 2008/042688 or WO 2009/014970.
The compound is described for example in WO 03/099836. Crystalline forms are described for example in WO 2008/002824.
The compound is described for example in EP 1354888 A1.
The compounds are described in EP 1 329 456 A1 and a crystalline form ofSergliflozin etabonate is described in EP 1 489 089 A1.
The compound is described in WO 2006/034489.
The compound (4-(Azulen-2-ylmethyl)-2-(β-D-glucopyranos-1-yl)-1-hydroxy-benzene) is described in WO 2004/013118 and WO 2006/006496. The crystalline choline salt thereof is described in WO 2007/007628.
The compound is described in WO 2004/080990 and WO 2005/012326. A cocrystal with L-proline is described in WO 2007/114475.
wherein R denotes methoxy or trifluoromethoxy. Such compounds and their method of production are described in WO 2004/007517, DE 102004063099 and WO 2006/072334.
The compound is described in WO 2005/012326. A crystalline hemihydrate is described in WO 2008/069327.
wherein R denotes methoxy, trifluoromethoxy, ethoxy, ethyl, isopropyl or tert. butyl. Such compounds are described in WO 2007/140191 and WO 2008/013280.
PATENTS
papers
Chinese Chemical Letters, 2013 , vol. 24, 2 pg. 131 – 133
Journal of Medicinal Chemistry, 2012 , vol. 55, 17 pg. 7828 – 7840
nmr
WO 2011074675

1 H-NMR (CD 3 OD) δ: 1.19 (3H, t, J = 7.5Hz), 2.59 (2H, q, J = 7.5Hz) ,3.42-3 .46 (1H , m), 3.65 (1H, dd, J = 5.5,12.0 Hz) ,3.74-3 .82 (4H, m), 3.96 (2H, s), 5.07 (1H , d, J = 12.8Hz), 5.13 (1H, d, J = 12.8Hz) ,7.08-7 .12 (4H, m) ,7.18-7 .23 (3H, m) .
MS (ESI +): 387 [M +1] +.
second set
http://pubs.acs.org/doi/full/10.1021/jm300884k
J. Med. Chem., 2012, 55 (17), pp 7828–7840
1H NMR (400 MHz, CD3OD) δ: 1.20 (3H, t, J = 7.6 Hz), 2.58 (2H, q, J = 7.6 Hz), 3.42–3.47 (1H, m), 3.63–3.67 (1H, m), 3.75–3.88 (4H, m), 3.95 (2H, s), 5.06 (1H, d, J = 12.3 Hz), 5.12 (1H, d, J = 12.5 Hz), 7.07–7.14 (4H, m), 7.17–7.23 (3H, m).
13C NMR (100 MHz, CD3OD) δ: 16.3, 29.4, 42.3, 62.8, 71.9, 73.4, 74.9, 76.2, 76.4, 111.6, 121.8, 123.6, 128.9, 129.9, 131.1, 139.7, 139.9, 140.2, 142.6, 143.2.
MS (ESI): 387 [M + H]+. HRMS (ESI), m/z calcd for C22H27O6 [M + H]+ 387.1802, found 387.1801.
……………………………
prepn
[Example 1] (1S, 3′R, 4′S, 5′S, 6′R) -6 – [(4 - ethyl-phenyl) methyl] -3 ‘, 4′, 5 ‘, 6′-tetrahydro- -6′-(hydroxymethyl) – spiro [isobenzofuran -1 (3H), 2'-[2H] pyran] -3 ‘, 4′, one of the preparation step [compound of formula (IX)] 5′-triol Preparation of methanol (2 – hydroxymethyl-phenyl – bromo-4)
To the mixing solution (1mol / L, 78.9kg, 88.4mol) of borane-tetrahydrofuran complex in tetrahydrofuran (6.34kg, 61.0mol) and, trimethoxyborane, two tetrahydrofuran (33.1kg) in – bromoterephthalic was added at below 30 ℃ solution (7.5kg, 30.6mol) of the acid, and the mixture was stirred for 1 hour at 25 ℃. Then cooled to 19 ℃ The reaction mixture was stirred for 30 minutes and added a mixed solution of tetrahydrofuran and methanol (3.0kg) of (5.6kg). In addition to methanol (15.0kg) in the mixture was kept for a while.
Again, to the mixing solution (1mol / L, 78.9kg, 88.4mol) of borane-tetrahydrofuran complex in tetrahydrofuran (6.34kg, 61.0mol) and, trimethoxyborane, two tetrahydrofuran (33.0kg) in – was added at below 30 ℃ solution (7.5kg, 30.6mol) of bromo terephthalic acid, and the reaction was carried out for 1 hour at 25 ℃. Then cooled to 18 ℃ The reaction mixture was stirred for 30 minutes and added a mixed solution of tetrahydrofuran and methanol (3.0kg) of (5.6kg). After addition of methanol (15.0kg) in the mixture is combined with the reaction mixture obtained in the previous reaction, and then the solvent was distilled off under reduced pressure. After addition of methanol (36kg) residue was obtained, and the solvent was evaporated under reduced pressure. Furthermore, (54 ℃ dissolved upon confirmation) which was dissolved by warming was added to methanol (36kg) to the residue. After cooling to room temperature the solution was stirred for 30 minutes added water (60kg). After addition of water (165kg) In addition to this mixture was cooled to 0 ℃, and the mixture was stirred for one hour. Centrifuge the obtained crystals were washed twice with water (45kg), and dried for 2 hours under reduced pressure to give (11.8kg, 54.4mol, 89% yield) of the title compound.
1 H-NMR (DMSO-d 6) δ: 4.49 (4H, t, J = 5.8Hz), 5.27 (1H, t, J = 5.8Hz), 5.38 (1H, t, J = 5.8Hz), 7.31 (1H, d, J = 7.5Hz), 7.47 (1H, d, J = 7.5Hz), 7.50 (1H, s).
Preparation of benzene (ethoxy methyl – methyl – - methoxy-1 1) – bromo-1 ,4 – 2:2 process bis
(- Bromo-4 – 2-hydroxyethyl methyl phenyl) in tetrahydrofuran (57kg) in the solution (8.0kg, 36.9mol) of methanol, I added (185.12g, 0.74mol) of pyridinium p-toluenesulfonate. After cooling to -15 ℃ below the mixture, 2 – was added at -15 ℃ or less (7.70kg, 106.8mol) methoxy propene, and the mixture was stirred 1 h at -15 ~ 0 ℃. Was added aqueous potassium carbonate (25 wt%, 40kg) and the reaction mixture was warmed to room temperature and separate the organic layer was added toluene (35kg). After washing with water (40kg) The organic layer was evaporated under reduced pressure. Was dissolved in toluene (28kg) and the residue obtained was obtained as a toluene solution of the title compound.
1 H-NMR (CDCl 3) δ: 1.42 (6H, s), 1.45 (6H, s), 3.24 (3H, s), 3.25 (3H, s), 4.45 ( 2H, s), 4.53 (2H, s), 7.28 (1H, dd, J = 1.5,8.0 Hz), 7.50 (1H, d, J = 8.0Hz), 7. 54 (1H, d, J = 1.5Hz).
MS (ESI +): 362 [M +2] +.
Preparation of on – (3R, 4S, 5R, 6R) -3,4,5 – tris (trimethylsilyloxy)-6 – trimethylsilyloxy methyl – tetrahydropyran-2: Step 3
Glucono -1,5 – - D-(+) in tetrahydrofuran (70kg) in the solution (35.8kg, 353.9mol) of N-methylmorpholine (7.88kg, 44.23mol) and lactone, chlorotrimethylsilane ( was added at 40 ℃ less 29.1kg, and 267.9mol), and the mixture was stirred for 2 hours at 30 ~ 40 ℃ resulting mixture. Was cooled to 0 ℃ the reaction mixture was added toluene (34kg) water (39kg), and the organic layer was separated. Twice sodium dihydrogen phosphate aqueous solution (5 wt%, 39.56kg) in, washed once with water (39kg) the organic layer the solvent was evaporated under reduced pressure. Was dissolved in toluene (34.6kg) and the residue obtained was obtained as a toluene solution of the title compound.
1 H-NMR (CDCl 3) δ: 0.13 (9H, s), 0.17 (9H, s), 0.18 (9H, s), 0.20 (9H, s), 3.74- 3.83 (3H, m), 3.90 (1H, t, J = 8.0Hz), 3.99 (1H, d, J = 8.0Hz), 4.17 (1H, dt, J = 2 .5,8.0 Hz).
Step 4: (1S, 3′R, 4′S, 5′S, 6′R) -3 ‘, 4′, 5 ‘, 6′-tetrahydro -6,6′ – bis (hydroxymethyl) – spiro [ (3H), 2'-[2H] pyran] -3 ‘, 4′, 5′-Preparation of triol isobenzofuran-1
(Methyl – - – methoxy 1-ethoxy-methyl) – bromo-1 ,4 – 2 prepared in step 2 bis cooled to below -10 ℃ toluene solution of benzene, hexane solution to (15 wt% n-butyl lithium , was added at below 0 ℃ 18.2kg, and 42.61mol), and the mixture was stirred 1.5 h at 5 ℃ resulting mixture. (10.5kg, 40.7mol), was added tetrahydrofuran (33.4kg) then magnesium bromide diethyl ether complex in the mixture, and the mixture was stirred for 1 hour at 25 ℃. Was added at below -10 ℃ toluene solution of the on – tris (trimethylsilyloxy) -6 – - 3,4,5 cooled to -15 ℃ below the mixture prepared in step 3 trimethylsilyloxy methyl – tetrahydropyran-2 was. After stirring 0.5 h at -15 ℃ or less, poured into 20% aqueous ammonium chloride solution to (80kg) of this solution, and the organic layer was separated. After washing with water (80kg) and the organic layer obtained, and the solvent was evaporated under reduced pressure. I was dissolved in methanol (43kg) residue was obtained. Was stirred for 1 hour at 20 ℃ was added (1.4kg, 7.4mol) and p-toluenesulfonic acid monohydrate in the mixture. Thereafter, it was stirred for another hour and cooled to 0 ℃, centrifuged crystals obtained was washed with methanol (25kg), and dried for 8 hours at reduced pressure under 40 ℃, (5.47kg, yield the title compound I got 50%) rate.
1 H-NMR (DMSO-d 6) δ :3.20-3 .25 (1H, m) ,3.41-3 .45 (1H, m) ,3.51-3 .62 (4H, m) , 4.39 (1H, t, J = 6.0Hz) ,4.52-4 .54 (3H, m), 4.86 (1H, d, J = 4.5Hz), 4.93 (1H, d, J = 5.5Hz), 4.99 (1H, d, J = 12.5Hz), 5.03 (1H, d, J = 12.5Hz), 5.23 (1H, t, J = 5 .8 Hz) ,7.24-7 .25 (2H, m), 7.29 (1H, dd, J = 1.5,8.0 Hz).
Step 5: (1S, 3′R, 4′S, 5′S, 6′R) -6 – [(methoxycarbonyl) methyl] -3 ‘, 4′, 5 ‘, 6′-tetrahydro-3′ , 4 ‘, 5′-tris (methoxycarbonyl) oxy-6′-[(methoxycarbonyl) methyl] – Preparation of [(3H), 2'-[2H] pyran isobenzofuran] spiro
(1S, 3′R, 4′S, 5′S, 6′R) – tetrahydro -6,6 ‘- bis (hydroxymethyl) – spiro [isobenzofuran -1 (3H), 2'-[2H] pyran ] -3 ‘, 4′, 5′-triol 4 (5.3kg, 17.8mol) and – dissolved in acetonitrile (35kg) (13.7kg, 112.1mol) a chloroformate, in the solution of dimethylaminopyridine I was added at 12 ℃ or less (10.01kg, 105.9mol) methyl. Heated to 20 ℃, After stirring for 1 h, was added ethyl acetate (40kg) and water (45kg), and the organic layer was separated and the mixture. Once (45.4kg) aqueous solution consisting of (9.01kg) sodium chloride and potassium hydrogen sulfate (1.35kg), sodium chloride aqueous solution (weight 10%, 44.5kg), sodium chloride aqueous solution (the organic layer was washed successively 20% by weight, in 45.0kg), and the solvent was evaporated under reduced pressure. Was dissolved in ethylene glycol dimethyl ether (18kg) and the residue obtained was then evaporated under reduced pressure. Was dissolved in ethylene glycol dimethyl ether (13.2kg) again and the residue obtained was obtained as ethylene glycol dimethyl ether solution of the title compound. I was used as it was in the six step.
1 H-NMR (CDCl 3) δ: 3.54 (3H, s), 3.77 (6H, s), 3.811 (3H, s), 3.812 (3H, s), 4.23 ( 1H, dd, J = 2.8,11.9 Hz), 4.32 (1H, dd, J = 4.0,11.9 Hz) ,4.36-4 .40 (1H, m), 5.11 -5.24 (5H, m), 5.41 (1H, d, J = 9.8Hz), 5.51 (1H, t, J = 9.8Hz), 7.25 (1H, d, J = 7.5Hz), 7.42 (1H, d, J = 7.5Hz), 7.44 (1H, s).
MS (ESI +): 589 [M +1] +, 606 [M +18] +.
Step 6: (1S, 3′R, 4′S, 5′S, 6′R) -6 – [(4 - ethyl-phenyl) methyl] -3 ‘, 4′, 5 ‘, 6′-tetrahydro-3 ’4′, 5′-tris (methoxycarbonyl) oxy-6′-[(methoxycarbonyl) methyl] – Preparation of [(3H), 2'-[2H] pyran isobenzofuran] spiro
[(Methoxycarbonyl) methyl] -3 ‘, 4′, 5 ‘, 6′-tetrahydro – (1S, 3′R, 4′S, 5′S, 6′R) -6 which had been prepared in Step 5 – 3 ‘, 4′, 5′-tris (methoxycarbonyl) oxy-6′-[(methoxycarbonyl) methyl] – spiro [isobenzofuran -1 (3H), 2'-[2H] pyran] Ethylene glycol dimethyl ether in solution, 2 – (2.46kg, 17.8mol), 4 butanol (25kg), anhydrous potassium carbonate – - methyl-2 were sequentially added (3.73kg, 24.9mol) ethyl phenyl boronic acid, in the reaction vessel was replaced with argon atmosphere, was bubbled with argon mixture. To the mixture – after the addition (0.72kg, 0.88mol) and palladium (II) chloride dichloromethane adduct [1,1 '-bis (diphenylphosphino) ferrocene], it was replaced with argon again inside of the vessel, one at 80 ℃ I was stirring time. After cooling, I added sequentially (0.859kg, 5.3mol) of ethylene glycol dimethyl ether (9.85kg), ethyl acetate (19kg), N-acetyl-L-cysteine in the mixture. After stirring for 2.5 h the mixture was filtered and added Celite (5.22kg), and washed with ethyl acetate (78kg) and the filter residue. The combined washings and filtrate, and the solvent is evaporated off under reduced pressure, and in addition (0.58kg, 3.6mol) and ethanol (74kg), N-acetyl-L-cysteine residue was obtained, which is heated to 70 ℃ or I was dissolved residue is then. After addition of water (9.4kg) in the solution, cooled to 60 ℃, and the mixture was stirred for 1 h. After confirming solid precipitated, cooled to 0 ℃ from 60 ℃ over 2.5 hours or more The mixture was stirred for 1 hour or more at 5 ℃ less. Centrifuge the resulting solid was washed twice with a mixture of water (35kg) and ethanol (55kg). Was dissolved at 70 ℃ ethanol (77kg) again, wet powder was obtained (10.21kg), cooled to 60 ℃ added water (9.7kg), and the mixture was stirred for 1 h. After confirming solid precipitated, cooled to 0 ℃ from 60 ℃ over 2.5 hours or more, and the mixture was stirred for 1 hour or more at 5 ℃ less. (9.45kg, dry powder rate 8.47kg, 13.7mol which was centrifuged obtained crystals were washed with a mixture of water (32kg) and ethanol (51kg), was obtained as a moist powder the title compound, 77% overall yield from the previous step).
1 H-NMR (CDCl 3) δ: 1.20 (3H, t, J = 7.5Hz), 2.60 (2H, q, J = 7.5Hz), 3.50 (3H, s), 3 .76 (3H, s), 3.77 (3H, s), 3.81 (3H, s), 3.96 (2H, s), 4.23 (1H, dd, J = 2.8,11 .9 Hz), 4.33 (1H, dd, J = 4.5,11.9 Hz) ,4.36-4 .40 (1H, m) ,5.11-5 .20 (3H, m), 5 .41 (1H, d, J = 10.0Hz), 5.51 (1H, t, J = 10.0Hz) ,7.07-7 .11 (4H, m), 7.14 (1H, d, J = 7.8Hz), 7.19 (1H, dd, J = 1.5,7.8 Hz), 7.31 (1H, d, J = 1.5Hz).
MS (ESI +): 619 [M +1] +, 636 [M +18] +.
Step 7: (1S, 3′R, 4′S, 5′S, 6′R) -6 – [(4 - ethyl-phenyl) methyl] -3 ‘, 4′, 5 ‘, 6′-tetrahydro-6 , 4 ‘, 5′-Preparation of triol’ – -3 [(3H), 2'-[2H] pyran isobenzofuran] spiro – (hydroxymethyl) ‘
(1S, 3′R, 4′S, 5′S, 6′R) -6 – [(4 - ethyl-phenyl) methyl] -3 ‘, 4′, 5 ‘, 6′-tetrahydro-3′, 4 ‘, 5′-tris (methoxycarbonyl) oxy-6′-[(methoxycarbonyl) methyl] – wet powder spiro [(3H), 2'-[2H] pyran isobenzofuran -1] (8.92kg, In addition at 20 ℃ (4mol / L, 30.02kg, the 104.2mol) aqueous solution of sodium hydroxide, 1 hour the reaction mixture to a solution of (28kg) ethylene glycol dimethyl ether dry end conversion 8.00kg, of 12.9mol) the mixture was stirred. And the organic layer was separated by addition of water (8.0kg) in the mixture. The ethyl acetate aqueous sodium chloride solution (25 wt%, 40kg) and a (36kg) in the organic layer and the aqueous layer was removed after washing. The washed again aqueous sodium chloride solution (25 wt%, 40kg) in the organic layer was evaporated under reduced pressure. Were added and acetone (32.0kg) water (0.8kg) residue was obtained. After the solvent was evaporated under reduced pressure, dissolved in acetone (11.7kg) in water (15.8kg) and the residue obtained was cooled to below 5 ℃. Was added below 10 ℃ water (64kg) to the mixture, and the mixture was stirred for 1 hour at below 10 ℃. Centrifuge the resulting crystals were washed with a mixture of water (8.0kg) and (1.3kg) acetone. For 8 hours through-flow drying 13 ~ 16 ℃ temperature ventilation, under the conditions of 24-33% relative humidity the wet powder, the monohydrate crystal (3.94kg, 9.7mol, 75% yield) of the title compound I was obtained as: (4.502 wt% water content).
Method of measuring the amount of water:
Analysis: coulometric KF titration analyzer: trace moisture measurement device manufactured by Mitsubishi Chemical Corporation Model KF-100
Anolyte: Aqua micron AX (manufactured by Mitsubishi Chemical Corporation)
Catholyte: Aqua micron CXU (manufactured by Mitsubishi Chemical Corporation)
1 H-NMR (CD 3 OD) δ: 1.19 (3H, t, J = 7.5Hz), 2.59 (2H, q, J = 7.5Hz) ,3.42-3 .46 (1H , m), 3.65 (1H, dd, J = 5.5,12.0 Hz) ,3.74-3 .82 (4H, m), 3.96 (2H, s), 5.07 (1H , d, J = 12.8Hz), 5.13 (1H, d, J = 12.8Hz) ,7.08-7 .12 (4H, m) ,7.18-7 .23 (3H, m) .
MS (ESI +): 387 [M +1] +.
………………………..
Example 1 Synthesis of 1,1-anhydro-1-C-[5-(4-ethylphenyl)methyl-2-(hydroxymethyl)phenyl]-β-D-glucopyranose Step 1: Synthesis of 3,4,5-tris(trimethylsilyloxy)-6-trimethylsilyloxymethyl-tetrahydropyran-2-one
To a solution of D-(+)-glucono-1,5-lactone (7.88 kg) and N-methylmorpholine (35.8 kg) in tetrahydrofuran (70 kg) was added trimethylsilyl chloride (29.1 kg) at 40° C. or below, and then the mixture was stirred at a temperature from 30° C. to 40° C. for 2 hours. After the mixture was cooled to 0° C., toluene (34 kg) and water (39 kg) were added thereto. The organic layer was separated and washed with an aqueous solution of 5% sodium dihydrogen phosphate (39.56 kg×2) and water (39 kg×1). The solvent was evaporated under reduced pressure to give the titled compound as an oil. The product was used in the next step without further purification.
1H-NMR (CDCl3) δ: 0.13 (9H, s), 0.17 (9H, s), 0.18 (9H, s), 0.20 (9H, s), 3.74-3.83 (3H, m), 3.90 (1H, t, J=8.0 Hz), 3.99 (1H, d, J=8.0 Hz), 4.17 (1H, dt, J=2.5, 8.0 Hz).
Step 2: Synthesis of 2,4-dibromo-1-(1-methoxy-1-methylethoxymethyl)benzene
Under a nitrogen atmosphere, to a solution of 2,4-dibromobenzyl alcohol (40 g, 0.15 mol) in tetrahydrofuran (300 ml) was added 2-methoxypropene (144 ml, 1.5 mol) at room temperature, and then the mixture was cooled to 0° C. At the same temperature, pyridinium p-toluenesulfonic acid (75 mg, 0.30 mmol) was added and the mixture was stirred for 1 hour. The reaction mixture was poured into a saturated aqueous solution of sodium hydrogen carbonate cooled to 0° C., and extracted with toluene. The organic layer was washed with a saturated aqueous solution of sodium chloride, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to give the titled compound as an oil in quantitative yield. The product was used in the next step without further purification.
1H-NMR (CDCl3) δ: 1.44 (6H, s), 3.22 (3H, 4.48 (2H, s), 7.42 (1H, d, J=8.0 Hz), 7.44 (1H, dd, J=1.5, 8.0 Hz), 7.68 (1H, d, J=1.5 Hz).
Step 3: Synthesis of 2,3,4,5-tetrakis(trimethylsilyloxy)-6-trimethylsilyloxymethyl-2-(5-(4-ethylphenyl)hydroxymethyl-2-(1-methoxy-1-methylethoxymethyl)phenyl)tetrahydropyran
Under a nitrogen atmosphere, 2,4-dibromo-1-(1-methoxy-1-methylethoxymethyl)benzene (70 g, 207 mmol), which was obtained in the previous step, was dissolved in toluene (700 mL) and t-butylmethyl ether (70 ml), and n-butyllithium in hexane (1.65 M, 138 ml, 227 mmol) was added dropwise at 0° C. over 30 minutes. After the mixture was stirred for 1.5 hours at 0° C., the mixture was added dropwise to a solution of 3,4,5-tris(trimethylsilyloxy)-6-trimethylsilyloxymethyl-tetrahydropyran-2-one (Example 1, 108 g, 217 mol) in tetrahydrofuran (507 ml) at −78° C., and the reaction mixture was stirred for 2 hours at the same temperature. Triethylamine (5.8 ml, 41 mmol) and trimethylsilyl chloride (29.6 ml, 232 mmol) were added thereto, and the mixture was warmed to 0° C. and stirred for 1 hour to give a solution containing 2,3,4,5-tetrakis(trimethylsilyloxy)-6-trimethylsilyloxymethyl-2-(5-bromo-2-(1-methoxy-1-methylethoxymethyl)phenyl)tetrahydropyran.
The resulting solution was cooled to −78° C., and n-butyllithium in hexane (1.65 M, 263 ml, 434 mmol) was added dropwise thereto at the same temperature. After the mixture was stirred at −78° C. for 30 minutes, 4-ethylbenzaldehyde (62 ml, 455 mmol) was added dropwise at −78° C., and the mixture was stirred at the same temperature for 2 hours. A saturated aqueous solution of ammonium chloride was added to the reaction mixture, and the organic layer was separated, and washed with water. The solvent was evaporated under reduced pressure to give a product containing the titled compound as an oil (238 g). The product was used in the next step without further purification.
A portion of the oil was purified by HPLC (column: Inertsil ODS-3, 20 mm I.D.×250 mm; acetonitrile, 30 mL/min) to give four diastereomers of the titled compound (two mixtures each containing two diastereomers).
Mixture of Diastereomers 1 and 2:
1H-NMR (500 MHz, CDCl3) δ: −0.47 (4.8H, s), −0.40 (4.2H, s), −0.003-0.004 (5H, m), 0.07-0.08 (1314, m), 0.15-0.17 (18H, m), 1.200 and 1.202 (3H, each t, J=8.0 Hz), 1.393 and 1.399 (3H, each s), 1.44 (3H, s), 2.61 (2H, q, J=8.0 Hz), 3.221 and 3.223 (3H, each s), 3.43 (1H, t, J=8.5 Hz), 3.54 (1H, dd, J=8.5, 3.0 Hz), 3.61-3.66 (1H, m), 3.80-3.85 (3H, m), 4.56 and 4.58 (1H, each d, J=12.4 Hz), 4.92 and 4.93 (1H, each d, J=12.4 Hz), 5.80 and 5.82 (1H, each d, J=3.0 Hz), 7.14 (2H, d, J=8.0 Hz), 7.28-7.35 (3H, m), 7.50-7.57 (2H, m).
MS (ESI+): 875 [M+Na]+.
Mixture of Diastereomers 3 and 4:
1H-NMR (500 MHz, toluene-d8, 80° C.) δ: −0.25 (4H, s), −0.22 (5H, s), 0.13 (5H, s), 0.16 (4H, s), 0.211 and 0.214 (9H, each s), 0.25 (9H, s), 0.29 (9H, s), 1.21 (3H, t, J=7.5 Hz), 1.43 (3H, s), 1.45 (3H, s), 2.49 (2H, q, J=7.5 Hz), 3.192 and 3.194 (3H, each s), 3.91-4.04 (4H, m), 4.33-4.39 (2H, m), 4.93 (1H, d, J=14.5 Hz), 5.10-5.17 (1H, m), 5.64 and 5.66 (1H, each s), 7.03 (2H, d, J=8.0 Hz), 7.28-7.35 (3H, m), 7.59-7.64 (1H, m), 7.87-7.89 (1H, m).
MS (ESI+): 875 [M+Na]+.
Step 4: Synthesis of 1,1-anhydro-1-C-[5-(4-ethylphenyl)hydroxymethyl-2-(hydroxymethyl)phenyl]-β-D-glucopyranose
Under a nitrogen atmosphere, the oil containing 2,3,4,5-tetrakis(trimethylsilyloxy)-6-trimethylsilyloxymethyl-2-(5-(4-ethylphenyl)hydroxymethyl-2-(1-methoxy-1-methylethoxymethyl)phenyl)tetrahydropyran (238 g), which was obtained in the previous step, was dissolved in acetonitrile (693 ml). Water (37 ml) and 1N HCl aq (2.0 ml) were added and the mixture was stirred at room temperature for 5.5 hours. Water (693 ml) and n-heptane (693 ml) were added to the reaction mixture and the aqueous layer was separated. The aqueous layer was washed with n-heptane (693 ml×2), and water was evaporated under reduced pressure to give a product containing water and the titled compound (a diastereomer mixture) as an oil (187 g). The product was used in the next step without further purification.
1H-NMR (500 MHz, CD3OD) δ: 1.200 (3H, t, J=7.7 Hz), 1.201 (3H, t, J=7.7 Hz), 2.61 (2H, q, J=7.7 Hz), 3.44-3.48 (1H, m), 3.63-3.68 (111, m), 3.76-3.84 (4H, m), 5.09 (1H, d, J=12.8 Hz), 5.15 (1H, d, J=12.8 Hz), 5.79 (1H, s), 7.15 (2H, d, J=7.7 Hz), 7.24 and 7.25 (1H, each d, J=8.4 Hz), 7.28 (2H, d, J=7.7 Hz), 7.36 (1H, dd, J=8.4, 1.5 Hz), 7.40-7.42 (114, m).
MS (ESI+): 425 [M+Na]+.
Step 5: Synthesis of 1,1-anhydro-1-C-[5-(4-ethylphenyl)methyl-2-(hydroxymethyl)phenyl]-β-D-glucopyranose (crude product)
To a solution of the oil containing 1,1-anhydro-1-C-[5-(4-ethylphenyl)hydroxymethyl-2-(hydroxymethyl)phenyl]-β-D-glucopyranose (187 g), which was obtained in the previous step, in 1,2-dimethoxyethane (693 ml) was added 5% Pd/C (26 g, 6.2 mmol, water content ratio: 53%), and the mixture was stirred in the atmosphere of hydrogen gas at room temperature for 4 hours. After filtration, the filtrate was evaporated under reduced pressure to give an oil containing the titled compound (59 g). The purity of the resulting product was 85.7%, which was calculated based on the area ratio measured by HPLC. The product was used in the next step without further purification.
1H-NMR (CD3OD) δ: 1.19 (3H, t, J=7.5 Hz), 2.59 (2H, q, J=7.5 Hz), 3.42-3.46 (1H, m), 3.65 (1H, dd, J=5.5, 12.0 Hz), 3.74-3.82 (4H, m), 3.96 (2H, s), 5.07 (1H, d, J=12.8 Hz), 5.13 (1H, d, J=12.8 Hz), 7.08-7.12 (4H, m), 7.18-7.23 (3H, m).
MS (ESI+): 387 [M+1]+.
Measurement Condition of HPLC:
Column: Cadenza CD-C18 50 mm P/NCD032
Mobile phase: Eluent A: H2O, Eluent B: MeCN
Gradient operation: Eluent B: 5% to 100% (6 min), 100% (2 min)
Flow rate: 1.0 mL/min
Temperature: 35.0° C.
Detection wavelength: 210 nm
Step 6: Synthesis of 1,1-anhydro-1-C-[5-(4-ethylphenyl)methyl-2-(hydroxymethyl)phenyl]-2,3,4,6-tetra-O-methoxycarbonyl-β-D-glucopyranose
Under a nitrogen atmosphere, to a solution of the oil containing 1,1-anhydro-1-C-[5-(4-ethylphenyl)methyl-2-(hydroxymethyl)phenyl]-β-D-glucopyranose (59 g) and 4-(dimethylamino)pyridine (175 g, 1436 mmol) in acetonitrile (1040 ml) was added dropwose methyl chloroformate (95 ml, 1231 mmol) at 0° C. The mixture was allowed to warm to room temperature while stirred for 3 hours. After addition of water, the mixture was extracted with isopropyl acetate. The organic layer was washed with an aqueous solution of 3% potassium hydrogensulfate and 20% sodium chloride (three times) and an aqueous solution of 20% sodium chloride, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure. To the resulting residue was added ethanol (943 mL) and the mixture was heated to 75° C. to dissolve the residue. The mixture was cooled to 60° C. and a seed crystal of the titled compound was added thereto. The mixture was cooled to room temperature and stirred for 1 hour. After precipitation of solid was observed, water (472 ml) was added thereto, and the mixture was stirred at room temperature for 2 hours. The resulting crystal was collected by filtration, washed with a mixture of water and ethanol (1:1), and dried under reduced pressure to give the titled compound (94 g). To the product (91 g) was added ethanol (1092 ml), and the product was dissolved by heating to 75° C. The solution was cooled to 60° C. and a seed crystal of the titled compound was added thereto. The mixture was cooled to room temperature and stirred for 1 hour. After precipitation of solid was observed, water (360 ml) was added thereto, and the mixture was stirred at room temperature for 2 hours. The resulting crystal was collected by filtration, washed with a mixture of water and ethanol (1:1), and dried under reduced pressure to give the titled compound [83 g, total yield from 2,4-dibromo-1-(1-methoxy-1-methylethoxymethyl)benzene used in Step 3: 68%].
1H-NMR (CDCl3) δ: 1.20 (3H, t, J=7.5 Hz), 2.60 (2H, q, J=7.5 Hz), 3.50 (3H, s), 3.76 (3H, s), 3.77 (3H, s), 3.81 (3H, s), 3.96 (2H, s), 4.23 (1H, dd, J=2.5, 11.8 Hz), 4.33 (1H, dd, J=4.5, 12.0 Hz), 4.36-4.40 (1H, m), 5.11-5.20 (3H, m), 5.41 (1H, d, J=10.0 Hz), 5.51 (1H, t, J=10.0 Hz), 7.07-7.11 (4H, m), 7.14 (1H, d, J=7.5 Hz), 7.19 (1H, dd, J=1.5, 7.8 Hz), 7.31 (1H, d, J=1.5 Hz).
MS (ESI+): 619 [M+1]+, 636 [M+18]+.
Another preparation was carried out in the same manner as Step 6, except that a seed crystal was not used, to give the titled compound as a crystal.
Step 7: Synthesis of 1,1-anhydro-1-C-[5-(4-ethylphenyl)methyl-2-(hydroxymethyl)phenyl]-β-D-glucopyranose
To a solution of 1,1-anhydro-1-C-[5-(4-ethylphenyl)methyl-2-(hydroxymethyl)phenyl]-2,3,4,6-tetra-O-methoxycarbonyl-β-D-glucopyranose (8.92 kg as wet powder, corresponding to 8.00 kg of dry powder) in 1,2-dimethoxyethane (28 kg) was added a solution of sodium hydroxide (4 mol/L, 30.02 kg) at 20° C., and the mixture was stirred for 1 hour. Water (8.0 kg) was added to the mixture and the layers were separated. To the organic layer were added an aqueous solution of 25% sodium chloride (40 kg) and ethyl acetate (36 kg). The organic layer was separated, washed with an aqueous solution of 25% sodium chloride (40 kg), and the solvent was evaporated under reduced pressure. The purity of the resulting residue was 98.7%, which was calculated based on the area ratio measured by HPLC. To the resulting residue were added acetone (32.0 kg) and water (0.8 kg), and the solvent was evaporated under reduced pressure. To the resulting residue were added acetone (11.7 kg) and water (15.8 kg), and the solution was cooled to 5° C. or below. Water (64 kg) was added to the solution at 10° C. or below, and the mixture was stirred at the same temperature for 1 hour. The resulting crystal was collected by centrifugation, and washed with a mixture of acetone (1.3 kg) and water (8.0 kg). The resulting wet powder was dried by ventilation drying under a condition at air temperature of 13 to 16° C. and relative humidity of 24% to 33% for 8 hours, to give a monohydrate crystal (water content: 4.502%) of the titled compound (3.94 kg). The purity of the resulting compound was 99.1%, which was calculated based on the area ratio measured by HPLC.
1H-NMR (CD3OD) δ: 1.19 (3H, t, J=7.5 Hz), 2.59 (2H, q, J=7.5 Hz), 3.42-3.46 (1H, m), 3.65 (1H, dd, J=5.5, 12.0 Hz), 3.74-3.82 (4H, m), 3.96 (2H, s), 5.07 (1H, d, J=12.8 Hz), 5.13 (1H, d, J=12.8 Hz), 7.08-7.12 (4H, m), 7.18-7.23 (311, m).
MS (ESI+): 387 [M+1]+.
Measurement Condition of HPLC:
Column: Capcell pack ODS UG-120 (4.6 mm I.D.×150 mm, 3 μm, manufactured by Shiseido Co., Ltd.)
Mobile phase: Eluent A: H2O, Eluent B: MeCN
Mobile phase sending: Concentration gradient was controlled by mixing Eluent A and Eluent B as indicated in the following table.
| TABLE 1 | ||||
| Time from | ||||
| injection (min) | Eluent A (%) | Eluent B (%) | ||
| 0 to 15 | 90→10 | 10→90 | ||
| 15 to 17.5 | 10 | 90 | ||
| 17.5 to 25 | 90 | 10 | ||
Flow rate: 1.0 mL/min
Temperature: 25.0° C.
Detection wavelength: 220 nm
Method for Measurement of Water Content:
Analysis method: coulometric titration method
KF analysis apparatus: Type KF-100 (trace moisture measuring apparatus manufactured by Mitsubishi Chemical Corporation)
Anode solution: Aquamicron AX (manufactured by Mitsubishi Chemical Corporation)
Cathode solution: Aquamicron CXU (manufactured by Mitsubishi Chemical Corporation)
…………………..
The compound of the present invention can be synthesized as shown in Scheme 1:
wherein R11 and R12 have the same meaning as defined above for substituents on Ar1, A is as defined above, and P represents a protecting group for a hydroxyl group.
REMOGLIFLOZIN ETABONATE
5-methyl-4-[4-(1-methylethoxy)benzyl]-1-(1-methylethyl)-1H-pyrazol-3-yl 6-O-(ethoxycarbonyl)-β-D-glucopyranoside, CAS 442201-24-3
189075 BHV-091009 GSK-189075 GSK-189075A KGT-1681
BHV Pharma Kissei (Originator) , GlaxoSmithKline
Remogliflozin etabonate (INN/USAN)[1] is a proposed drug for the treatment of type 2diabetes being investigated by GlaxoSmithKline.[2] Remogliflozin is now being developed by BHV Pharma.
Remogliflozin inhibits the sodium-glucose transport proteins, which are responsible for glucose reabsorption in the kidney. Blocking this transporter causes blood glucose to be eliminated through the urine.[3]
DPP IV inhibitors represent a novel class of agents that are being developed for the treatment or improvement in glycemic control in patients with type 2 diabetes. For example, DPP IV inhibitors and their uses are disclosed in WO 2002/068420, WO 2004/018467, WO 2004/018468, WO 2004/018469, WO 2004/041820, WO 2004/046148, WO 2005/051950, WO 2005/082906, WO 2005/063750, WO 2005/085246, WO 2006/027204, WO 2006/029769, WO2007/014886; WO 2004/050658, WO 2004/1 1 1051 , WO 2005/058901 , WO 2005/097798; WO 2006/068163, WO 2007/071738, WO 2008/017670; WO 2007/054201 or WO 2007/128761.

Chemical structures of remogliflozin etabonate (A), remogliflozin (B), sergliflozin (C), phlorizin (D), and T-1095 (E). Remogliflozin etabonate is metabolized to remogliflozin, its active form.

LUSEOGLIFLOZIN, CAS 898537-18-3
An antidiabetic agent that inhibits sodium-dependent glucose cotransporter 2 (SGLT2).
Taisho (Originator), PHASE 3
TS-071
TS-071, an SGLT-2 inhibitor, is in phase III clinical development at Taisho for the oral treatment of type 1 and type 2 diabetes
In 2012, the product was licensed to Novartis and Taisho Toyama Pharmaceutical by Taisho in Japan for comarketing for the treatment of type 2 diabetes.
Diabetes is a metabolic disorder which is rapidly emerging as a global health care problem that threatens to reach pandemic levels. The number of people with diabetes worldwide is expected to rise from 285 million in 2010 to 438 million by 2030. Diabetes results from deficiency in insulin because of impaired pancreatic β-cell function or from resistance to insulin in body, thus leading to abnormally high levels of blood glucose.
Diabetes which results from complete deficiency in insulin secretion is Type 1 diabetes and the diabetes due to resistance to insulin activity together with an inadequate insulin secretion is Type 2 diabetes. Type 2 diabetes (Non insulin dependent diabetes) accounts for 90-95 % of all diabetes. An early defect in Type 2 diabetes mellitus is insulin resistance which is a state of reduced responsiveness to circulating concentrations of insulin and is often present years before clinical diagnosis of diabetes. A key component of the pathophysiology of Type 2 diabetes mellitus involves an impaired pancreatic β-cell function which eventually contributes to decreased insulin secretion in response to elevated plasma glucose. The β-cell compensates for insulin resistance by increasing the insulin secretion, eventually resulting in reduced β-cell mass. Consequently, blood glucose levels stay at abnormally high levels (hyperglycemia).
Hyperglycemia is central to both the vascular consequences of diabetes and the progressive nature of the disease itself. Chronic hyperglycemia leads to decrease in insulin secretion and further to decrease in insulin sensitivity. As a result, the blood glucose concentration is increased, leading to diabetes, which is self-exacerbated. Chronic hyperglycemia has been shown to result in higher protein glycation, cell apoptosis and increased oxidative stress; leading to complications such as cardiovascular disease, stroke, nephropathy, retinopathy (leading to visual impairment or blindness), neuropathy, hypertension, dyslipidemia, premature atherosclerosis, diabetic foot ulcer and obesity. So, when a person suffers from diabetes, it becomes important to control the blood glucose level. Normalization of plasma glucose in Type 2 diabetes patients improves insulin action and may offset the development of beta cell failure and diabetic complications in the advanced stages of the disease.
Diabetes is basically treated by diet and exercise therapies. However, when sufficient relief is not obtained by these therapies, medicament is prescribed alongwith. Various antidiabetic agents being currently used include biguanides (decrease glucose production in the liver and increase sensitivity to insulin), sulfonylureas and meglitinides (stimulate insulin production), a-glucosidase inhibitors (slow down starch absorption and glucose production) and thiazolidinediones (increase insulin sensitivity). These therapies have various side effects: biguanides cause lactic acidosis, sulfonylurea compounds cause significant hypoglycemia, a-glucosidase inhibitors cause abdominal bloating and diarrhea, and thiazolidinediones cause edema and weight gain. Recently introduced line of therapy includes inhibitors of dipeptidyl peptidase-IV (DPP-IV) enzyme, which may be useful in the treatment of diabetes, particularly in Type 2 diabetes. DPP-IV inhibitors lead to decrease in inactivation of incretins glucagon like peptide- 1 (GLP-1) and gastric inhibitory peptide (GIP), thus leading to increased production of insulin by the pancreas in a glucose dependent manner. All of these therapies discussed, have an insulin dependent mechanism.
Another mechanism which offers insulin independent means of reducing glycemic levels, is the inhibition of sodium glucose co-transporters (SGLTs). In healthy individuals, almost 99% of the plasma glucose filtered in the kidneys is reabsorbed, thus leading to only less than 1% of the total filtered glucose being excreted in urine. Two types of SGLTs, SGLT-1 and SGLT-2, enable the kidneys to recover filtered glucose. SGLT-1 is a low capacity, high-affinity transporter expressed in the gut (small intestine epithelium), heart, and kidney (S3 segment of the renal proximal tubule), whereas SGLT-2 (a 672 amino acid protein containing 14 membrane-spanning segments), is a low affinity, high capacity glucose ” transporter, located mainly in the S 1 segment of the proximal tubule of the kidney. SGLT-2 facilitates approximately 90% of glucose reabsorption and the rate of glucose filtration increases proportionally as the glycemic level increases. The inhibition of SGLT-2 should be highly selective, because non-selective inhibition leads to complications such as severe, sometimes fatal diarrhea, dehydration, peripheral insulin resistance, hypoglycemia in CNS and an impaired glucose uptake in the intestine.
Humans lacking a functional SGLT-2 gene appear to live normal lives, other than exhibiting copious glucose excretion with no adverse effects on carbohydrate metabolism. However, humans with SGLT-1 gene mutations are unable to transport glucose or galactose normally across the intestinal wall, resulting in condition known as glucose-galactose malabsorption syndrome.
Hence, competitive inhibition of SGLT-2, leading to renal excretion of glucose represents an attractive approach to normalize the high blood glucose associated with diabetes. Lower blood glucose levels would, in turn, lead to reduced rates of protein glycation, improved insulin sensitivity in liver and peripheral tissues, and improved cell function. As a consequence of progressive reduction in hepatic insulin resistance, the elevated hepatic glucose output which is characteristic of Type 2 diabetes would be expected to gradually diminish to normal values. In addition, excretion of glucose may reduce overall caloric load and lead to weight loss. Risk of hypoglycemia associated with SGLT-2 inhibition mechanism is low, because there is no interference with the normal counter regulatory mechanisms for glucose.
The first known non-selective SGLT-2 inhibitor was the natural product phlorizin
(glucose, 1 -[2-P-D-glucopyranosyloxy)-4,6-dihydroxyphenyl]-3-(4-hydroxyphenyl)- 1 – propanone). Subsequently, several other synthetic analogues were derived based on the structure of phlorizin. Optimisation of the scaffolds to achieve selective SGLT-2 inhibitors led to the discovery of several considerably different scaffolds.
C-glycoside derivatives have been disclosed, for example, in PCT publications
W.O20040131 18, WO2005085265, WO2006008038, WO2006034489, WO2006037537, WO2006010557, WO2006089872, WO2006002912, WO2006054629, WO2006064033, WO2007136116, WO2007000445, WO2007093610, WO2008069327, WO2008020011, WO2008013321, WO2008013277, WO2008042688, WO2008122014, WO2008116195, WO2008042688, WO2009026537, WO2010147430, WO2010095768, WO2010023594, WO2010022313, WO2011051864, WO201 1048148 and WO2012019496 US patents US65151 17B2, US6936590B2 and US7202350B2 and Japanese patent application JP2004359630. The compounds shown below are the SGLT-2 inhibitors which have reached advanced stages of human clinical trials: Bristol-Myers Squibb’s “Dapagliflozin” with Formula A, Mitsubishi Tanabe and Johnson & Johnson’s “Canagliflozin” with Formula B, Lexicon’s “Lx-421 1″ with Formula C, Boehringer Ingelheim and Eli Lilly’s “Empagliflozin” with Formula D, Roche and Chugai’s “Tofogliflozin” with Formula E, Taisho’s “Luseogliflozin” with Formula F, Pfizer’ s “Ertugliflozin” with Formula G and Astellas and Kotobuki’s “Ipragliflozin” with Formula H.
Formula G Formula H
In spite of all these molecules in advanced stages of human clinical trials, there is still no drug available in the market as SGLT-2 inhibitor. Out of the potential candidates entering the clinical stages, many have been discontinued, emphasizing the unmet need. Thus there is an ongoing requirement to screen more scaffolds useful as SGLT-2 inhibitors that can have advantageous potency, stability, selectivity, better half-life, and/ or better pharmacodynamic properties. In this regard, a novel class of SGLT-2 inhibitors is provided herein
SYNTHESIS
Synthesis of 2,3,4,6-tetra-O-benzyl-1-C-[2-methoxy-4-methyl-(4-ethoxybenzyl)phenyl]-5-thio-D-glucopyranose
Example 6
Synthesis of (1S)-1,5-anhydro-2,3,4,6-tetra-O-benzyl-1-[2-methoxy-4-methyl-5-(4-ethoxybenzyl)phenyl]-1-thio-D-glucitol
Example 7
Synthesis of (1S)-1,5-anhydro-1-[3-(4-ethoxybenzyl)-6-methoxy-4-methylphenyl]-1-thio-D-glucitol
 Small synthetic molecule identified that ameliorates obesity-related disorders in animal models
Small synthetic molecule identified that ameliorates obesity-related disorders in animal models