Green Chem., 2015, Advance Article
DOI: 10.1039/C4GC02033B, Paper
Jiayin Hu, Jun Ma, Zhaofu Zhang, Qinggong Zhu, Huacong Zhou, Wenjing Lu, Buxing Han
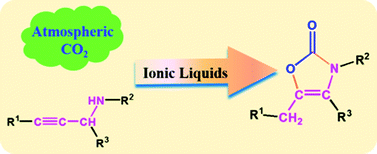
CO2 can react with various propargylic amines to form 3,4,5-trisubstituted oxazolones catalyzed by the active, selective and stable ionic liquids.
Production of value-added chemicals using carbon dioxide (CO2) as a feedstock is favorable to the sustainable development of the chemical industry. In this work, we have discovered for the first time that CO2 can react with propargylic amines to produce 3,4,5-trisubstituted oxazolones, a class of very useful chemicals. It was found that the ionic liquid (IL) 1-butyl-3-methylimidazolium acetate ([Bmim][OAc]) can catalyze the reactions efficiently at atmospheric pressure under metal-free conditions. It was also found that [Bmim][OAc] and IL 1-butyl-3-methylimidazolium bis((trifluoromethyl)sulfonyl)imide ([Bmim][Tf2N]) have an excellent synergistic effect for promoting the reactions. The [Bmim][OAc]/[Bmim][Tf2N] catalytic system can be reused at least five times without loss in catalytic activity and selectivity. The reaction mechanism was proposed on the basis of density functional theory (DFT) calculation and the experimental results.