Clik here to view.
 Image may be NSFW.
Image may be NSFW.Clik here to view.
 Image may be NSFW.
Image may be NSFW.Clik here to view.
 Image may be NSFW.
Image may be NSFW.Clik here to view.
 Image may be NSFW.
Image may be NSFW.Clik here to view.
 Image may be NSFW.
Image may be NSFW.Clik here to view.
 Image may be NSFW.
Image may be NSFW.Clik here to view.
 Image may be NSFW.
Image may be NSFW.Clik here to view.
 Image may be NSFW.
Image may be NSFW.Clik here to view.
 Image may be NSFW.
Image may be NSFW.Clik here to view.
 Image may be NSFW.
Image may be NSFW.Clik here to view.
 Image may be NSFW.
Image may be NSFW.Clik here to view.

Image may be NSFW.
Clik here to view.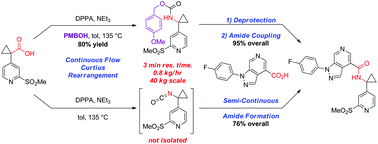
Development of a concise, scalable synthesis of a CCR1 antagonist utilizing a continuous flow Curtius rearrangement
DOI: 10.1039/C6GC03123D, Paper
A convergent and robust synthesis of a developmental CCR1 antagonist is described using continuous flow technology
A convergent, robust, and concise synthesis of a developmental CCR1 antagonist is described using continuous flow technology. In the first approach, following an expeditious SNAr sequence for cyclopropane introduction, a safe, continuous flow Curtius rearrangement was developed for the synthesis of a p-methoxybenzyl (PMB) carbamate. Based on kinetic studies, a highly efficient and green process comprising three chemical transformations (azide formation, rearrangement, and isocyanate trapping) was developed with a relatively short residence time and high material throughput (0.8 kg h−1, complete E-factor = ∼9) and was successfully executed on 40 kg scale. Moreover, mechanistic studies enabled the execution of a semi-continuous, tandem Curtius rearrangement and acid–isocyanate coupling to directly afford the final drug candidate in a single, protecting group-free operation. The resulting API synthesis is further determined to be extremely green (RPG = 166%) relative to the industrial average for molecules of similar complexity.
Development of a concise, scalable synthesis of a CCR1 antagonist utilizing a continuous flow Curtius rearrangement
E-mail: maurice.marsini@boehringer-ingelheim.com
DOI: 10.1039/C6GC03123D
Image may be NSFW.
Clik here to view.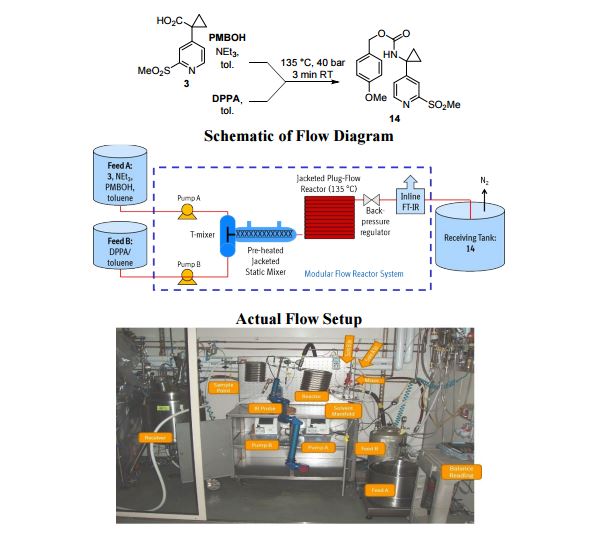 Image may be NSFW.
Image may be NSFW.
Clik here to view.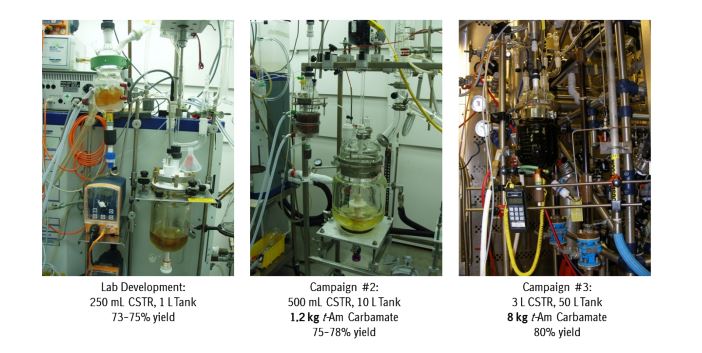 Image may be NSFW.
Image may be NSFW.
Clik here to view.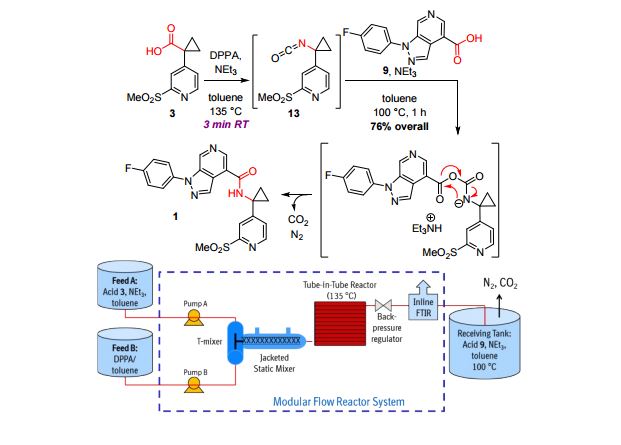
Image may be NSFW.
Clik here to view.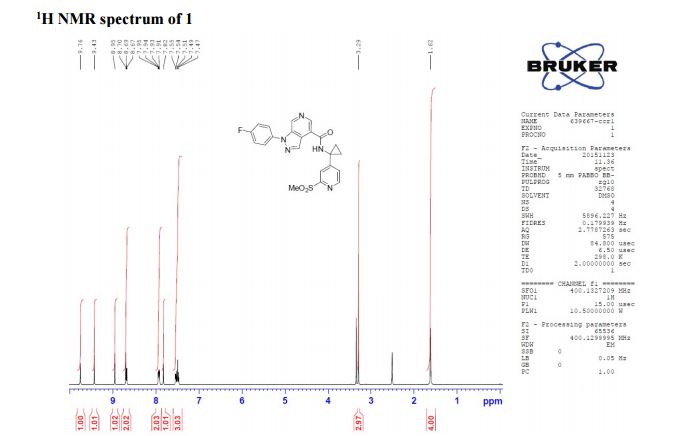 Image may be NSFW.
Image may be NSFW.
Clik here to view.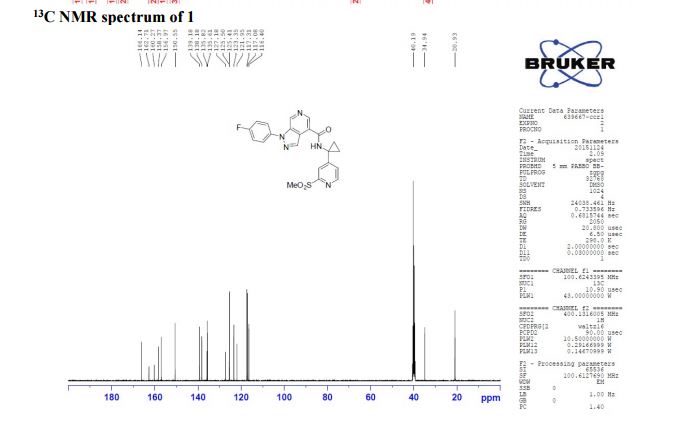
1-(4-fluorophenyl)-N-(1-(2-(methylsulfonyl)pyridin-4-yl)cyclopropyl)-1H-pyrazolo[3,4- c]pyridine-4-carboxamide
1-(4-fluorophenyl)-N-(1-(2-(methylsulfonyl)pyridin-4-yl)cyclopropyl)-1H-pyrazolo[3,4- c]pyridine-4-carboxamide
m.p. = 140-144 °C;
1H NMR (400 MHz, CDCl3) δ 9.76 (s, 1H), 9.43 (s, 1H), 8.95 (s, 1H), 8.70 (s, 1H), 8.68 (d, J = 5.2 Hz, 1H), 7.93 (s, J1 = 8.8 Hz, J2 = 4.7 Hz, 1H), 7.82 (s, 1H), 7.54 (d, J = 4.1 Hz, 1H), 7.49 (t, J = 8.7 Hz, 1H), 3.29 (s, 3H), 1.61 (bs, 4H);
13C NMR (100 MHz, CDCl3) δ 166.1, 162.7, 160.3, 158.4, 156.9, 150.6, 139.2, 138.2, 135.8, 135.6, 125.4 (d, JC-F = 8.8 Hz), 123.3, 121.9, 117.2 (d, JC-F = 23.1 Hz), 116.4, 40.2, 34.9, 20.9;
HRMS: calcd for C22H19FN5O3S [M + H+ ]: 452.1187. Found: 452.1189.
Image may be NSFW.
Clik here to view.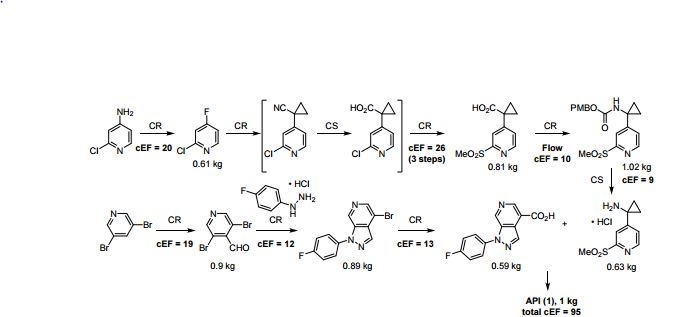
Image may be NSFW.
Clik here to view.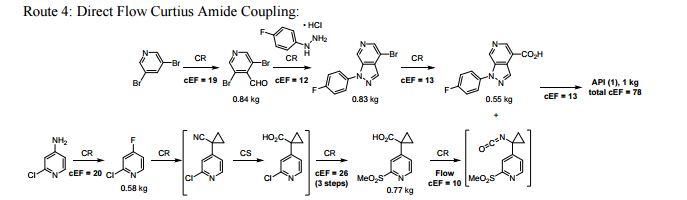
Image may be NSFW.
Clik here to view.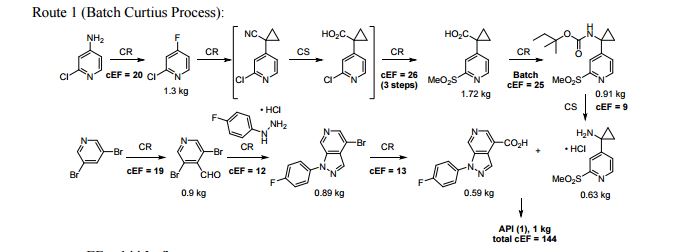
Image may be NSFW.
Clik here to view.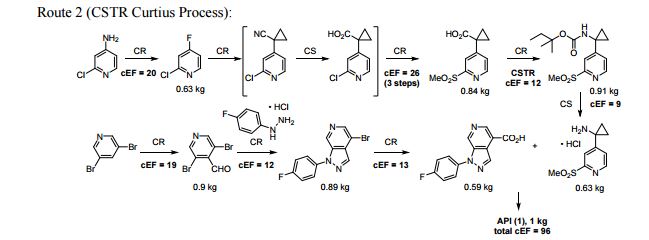
//////////